New Droplet-on-Tape Method Assists Biochemical Research at X-Ray Lasers
A research collaboration designed a new assembly-line system that rapidly replaces exposed samples and allows the team to study reactions in real-time.
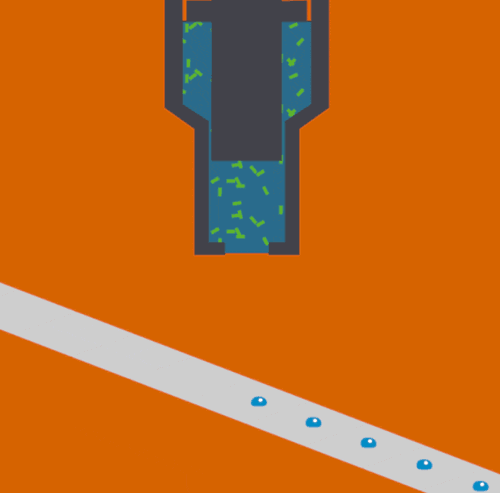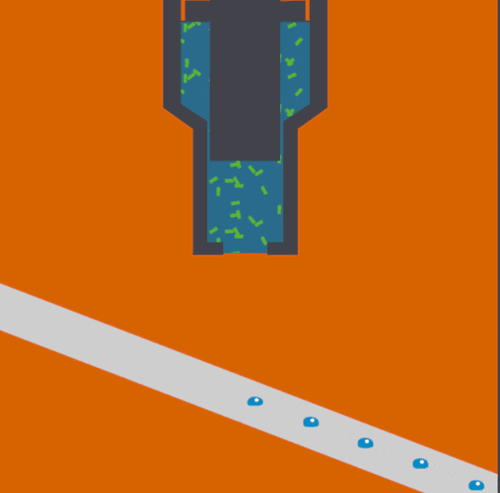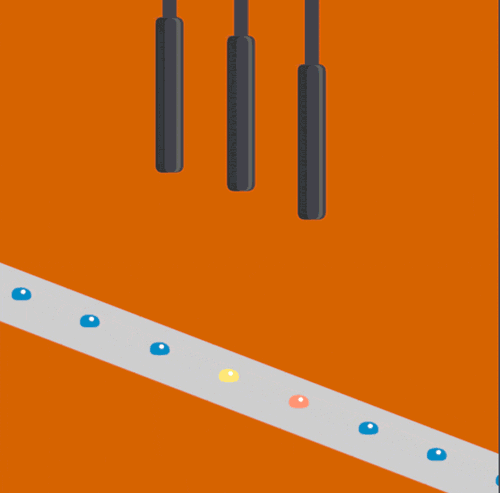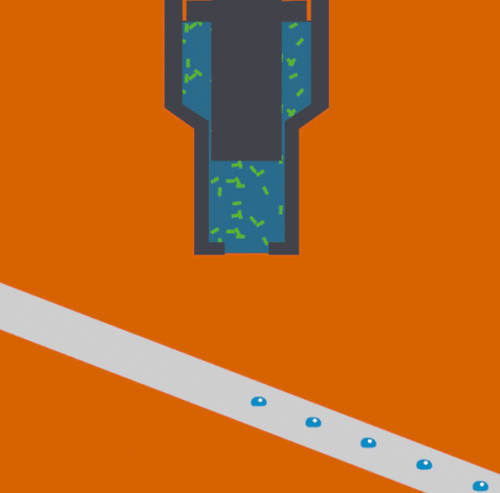
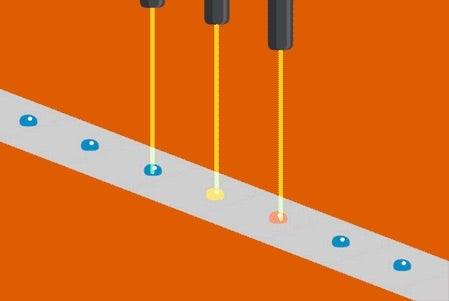
Biological samples studied with intense X-rays at free-electron lasers are destroyed within nanoseconds after they are exposed. Because of this, the samples need to be continually refreshed to allow the many images needed for an experiment to be obtained. Conventional methods use jets that supply a continuous stream of samples, but this can be very wasteful as the X-rays only interact with a tiny fraction of the injected material.
To help address this issue, scientists at the Department of Energy's Lawrence Berkeley National Laboratory, SLAC National Accelerator Laboratory, Brookhaven National Laboratory, and other institutes designed a new assembly-line system that rapidly replaces exposed samples by moving droplets along a miniature conveyor belt, timed to coincide with the arrival of the X-ray pulses. The droplet-on-tape system now allows the team to study the biochemical reactions in real-time from microseconds to seconds, revealing the stages of these complex reactions.
In their approach, protein solution or crystals are precisely deposited in tiny liquid drops, made as ultrasound waves push the liquid onto a moving tape. As the drops move forward, they are hit with pulses of visible light or treated with oxygen gas, which triggers different chemical reactions depending on the sample studied. This allows the study of processes such as photosynthesis, which determines how plants absorb light from the sun and convert it into useable energy.
Finally, powerful X-ray pulses from SLAC’s X-ray laser, the Linac Coherent Light Source (LCLS), probe the drops. In this study published in Nature Methods, the X-ray light scattered from the sample onto two different detectors simultaneously, one for X-ray crystallography and the other for X-ray emission spectroscopy. These are two complementary methods that provide information about the geometric and electronic structure of the catalytic sites of the proteins and allowed them to watch with atomic precision how the protein structures changed during the reaction.
Below, see the conveyor belt in action at LCLS, a Department of Energy Office of Science User Facility.
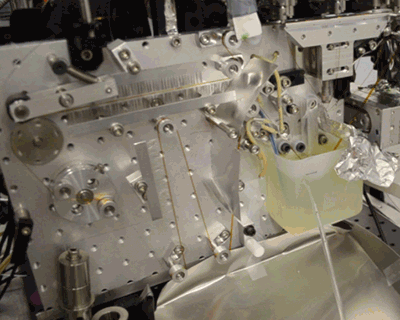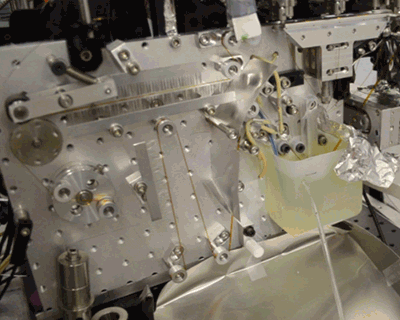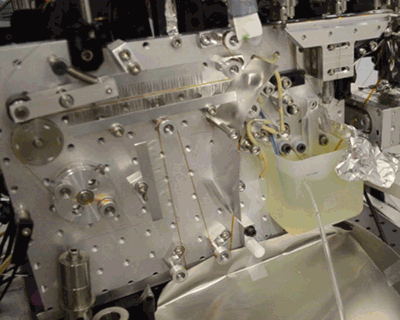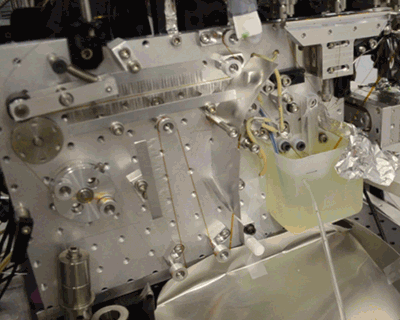
Citation
Fuller et al., Nature Methods, 27 February 2017 (10.1038/nmeth.4195)
Contact
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.