Attosecond pulses reveal electronic ripples in molecules
Researchers demonstrate a new ability to drive and track electronic motion, which is crucial to understanding the role of electrons in chemical processes and how quantum coherence evolves on the shortest timescales.
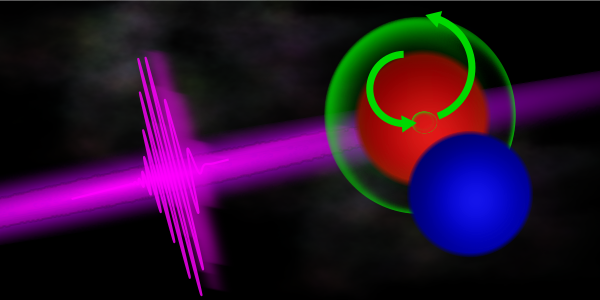
In the first experiment to take advantage of a new technology for producing powerful attosecond X-ray laser pulses, a research team led by scientists from the Department of Energy’s SLAC National Accelerator Laboratory and Stanford University showed they can create electronic ripples in molecules through a process called “impulsive Raman scattering.”
Exploiting this unique interaction will allow scientists to study how electrons zipping around molecules kick off key processes in biology, chemistry, materials science and more. The researchers described their results in Physical Review Letters.
Typically, when X-ray pulses interact with matter the X-rays cause the molecules’ innermost “core” electrons to jump to higher energies. These core-excited states are highly unstable, decaying in just millionths of a billionth of a second. In a majority of X-ray experiments, that’s how the story ends: The excited electrons quickly return to their rightful places by transferring their energy to a neighboring electron, forcing it out of the atom and producing a charged ion.
However, with a sufficiently short and intense X-ray pulse, the atom can be forced to respond differently, opening up new ways to measure and control matter. The X-rays can excite the core electron but then also drive an outlying electron to fill the gap. This allows the molecule to enter an excited state while keeping its atoms in a stable, neutral state. Since this Raman process relies on core-level electrons, the electronic excitation is initially highly localized in the molecule, making it easier to pinpoint its origin and track its evolution.
“If you think of the molecule's electrons as a lake, the Raman interaction is similar to taking a rock and tossing it into the water,” says co-author and SLAC scientist James Cryan. “This ‘excitation’ creates waves that ripple across the surface from a specific point. In a similar way, X-ray excitations create ‘charge waves’ that ripple across the molecule. They provide researchers with an entirely new way to measure the response of a molecule to light.”
Pulses of visible light can also be used to create excited state molecules, but those pulses are more like a small earthquake that ripples the entire surface of the water. The impulsive Raman X-ray excitation gives much more information about the properties of the molecule, the equivalent of dropping rocks in various places to produce and observe different ripple patterns.
Earlier LCLS experiments demonstrated the Raman process in atoms, but until now observing this process in molecules has evaded scientists. This experiment succeeded because of recent developments in producing X-ray free-electron laser (FEL) pulses 10 to 100 times shorter than before. Led by SLAC scientist Agostino Marinelli, the X-ray Laser-Enhanced Attosecond Pulse project (XLEAP) provided a method to generate intense pulses that are just 280 attoseconds, or billionths of a billionth of a second, long. These pulses were critical to the success of the experiment and will allow scientists to jumpstart chemical reactions and coherent quantum processes in the future.
“This experiment showcases the unique properties of attosecond FELs compared to state of the art laser-based attosecond sources,” Marinelli says. “Most importantly, this experiment shows how close collaboration between accelerator scientists and the user community can lead to exciting new science.”
LCLS is a DOE Office of Science user facility. This research was supported by the Office of Science.)
Citation: J.T. O'neal et al., Physical Review Letters, 11 August 2020 (10.1103/PhysRevLett.125.073203)
Contact
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
About SLAC
SLAC National Accelerator Laboratory explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by researchers around the globe. As world leaders in ultrafast science and bold explorers of the physics of the universe, we forge new ground in understanding our origins and building a healthier and more sustainable future. Our discovery and innovation help develop new materials and chemical processes and open unprecedented views of the cosmos and life’s most delicate machinery. Building on more than 60 years of visionary research, we help shape the future by advancing areas such as quantum technology, scientific computing and the development of next-generation accelerators.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.





