Scientists capture the fleeting transition of water into a highly reactive state
This is the first direct observation of a hydroxyl-hydronium complex – important for a wide range of chemical and biological processes from the tails of comets to cancer treatment.
Researchers at the Department of Energy’s SLAC National Accelerator Laboratory have uncovered a key step in the ionization of liquid water using the lab’s high-speed “electron camera,” MeV-UED. This reaction is of fundamental significance to a wide range of fields, including nuclear engineering, space travel, cancer treatment and environmental remediation. Their results were published in Science today.
When high-energy radiation hits a water molecule, it triggers a series of ultrafast reactions. First, it kicks out an electron, leaving behind a positively charged water molecule. Within a fraction of a trillionth of a second, this water molecule gives up a proton to another water molecule. This leads to the creation of a hydroxyl radical (OH) – which can damage virtually any macromolecule in an organism, including DNA, RNA and proteins – and a hydronium ion (H3O+), which are abundant in the interstellar medium and tails of comets, and might contain clues about the origin of life.
Capturing the unstable pair
In a previous Science paper published in 2020, a team led by scientists at the DOE’s Argonne National Laboratory used SLAC’s Linac Coherent Light Source (LCLS) X-ray laser to witness, for the first time, the ultrafast proton transfer reaction following ionization of liquid water. But until now, researchers had yet to directly observe the hydroxyl-hydronium pair.
“All laser surgeries and radiotherapies produce this unstable complex, which may lead to many chemical reactions in the human body,” says SLAC scientist and study lead Ming-Fu Lin. “Interestingly, this complex also helps to purify our drinking water by killing germs. It is also of importance in nuclear power generation where water is ionized by other forms of radiation. Many simulations predict the existence of this complex but now we have finally observed its formation.”
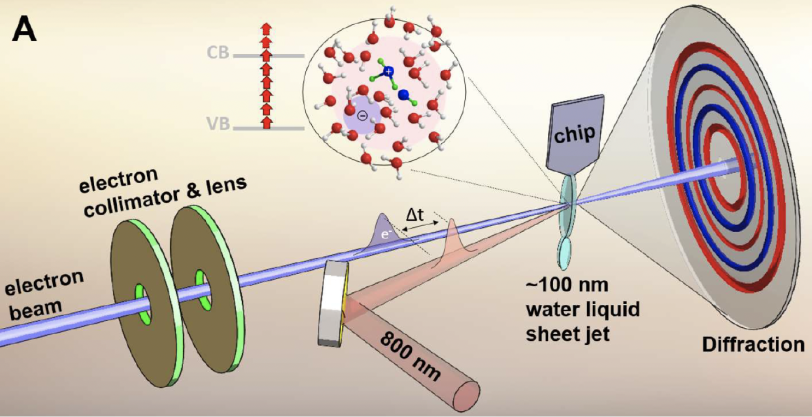
To observe the short-lived hydroxyl-hydronium pair, the researchers created 100-nanometer-thick jets of liquid water – about 1,000 times thinner than the width of a human hair – and ionized the water molecules with intense laser light. Then they probed the molecules with short pulses of high-energy electrons from MeV-UED to generate high-resolution snapshots of the ionization process. This allowed them to measure bonds between oxygen atoms and bonds between oxygen and hydrogen atoms at the same time, thus capturing this important but unstable complex.
Opening a window on chemical reactions
To follow up, the researchers plan to increase the imaging speed so the proton transfer process can be measured directly prior to the formation of the hydroxyl-hydronium pairs. They also hope to observe the ejected electron in the liquid water to better understand how it affects the process.
“Both topics have been intensively studied by simulations, but no direct structural measurements have been taken to validate theories,” says Matthias Ihme, an associate professor in the Stanford University Mechanical Engineering department who led the theoretical analysis. “These measurements are also critical for testing our theoretical models that predict these processes.”
“Many intermediate states and structures in chemical reactions are either unknown or have yet to be observed directly,” adds Xijie Wang, a SLAC distinguished staff scientist and study collaborator. “We can use MeV-UED to explore and capture various short-lived and important complexes, opening a window to study chemical reactions as they occur.”
MeV-UED is an instrument of the LCLS user facility, operated by SLAC on behalf of the DOE Office of Science, which funded this research.
Citation: M.F. Lin et al., Science, 30 Sep 2021 (10.1126/science.abg3091)
Contact
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a vibrant multiprogram laboratory that explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by scientists around the globe. With research spanning particle physics, astrophysics and cosmology, materials, chemistry, bio- and energy sciences and scientific computing, we help solve real-world problems and advance the interests of the nation.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.





