Scientists Make First Observations of How a Meteor-Like Shock Turns Silica Into Glass
Research with SLAC’s X-ray laser simulates what happens when a meteor hits Earth’s crust. The results suggest that scientists studying impact sites have been overestimating the sizes of the meteors that made them.
By Julia Goldstein
Studies at the Department of Energy’s SLAC National Accelerator Laboratory have made the first real-time observations of how silica – an abundant material in the Earth’s crust – easily transforms into a dense glass when hit with a massive shock wave like one generated from a meteor impact.
The results imply that meteors hitting Earth and other celestial objects are smaller than originally thought. This new information will be important for modeling planetary body formation and interpreting evidence of impacts on the ground.
The experiments took place at SLAC’s Linac Coherent Light Source (LCLS) X-ray laser, a DOE Office of Science User Facility whose ultrafast pulses can reveal processes taking place in millionths of a billionth of a second with atomic resolution.
“We were able for the first time to really visualize from start to finish what happens in a material that makes up a major portion of the Earth’s crust,” said Arianna Gleason of the DOE’s Los Alamos National Laboratory (LANL), the principal investigator for the study, which was published Nov. 14 in Nature Communications.

How Does Shocked Glass Get That Way?
Scientists have long known that impacts from meteors convert silicates into a dense, amorphous phase known as shocked glass. The question is how this shocked glass forms.
In the past, scientists have tried to estimate the amount of pressure needed to cause this transformation by examining debris from meteor impacts and squeezing mineral samples in pressure cells in the lab, but they were unable to observe the process as it unfolded.
At LCLS, researchers can use an intense laser beam to create a shock wave that compresses a silica sample, and then use the X-ray laser to examine its response on a timescale of nanoseconds, or billionths of a second.
A previous SLAC study, published in 2015, demonstrated that silica forms stishovite, a crystalline phase, within 10 nanoseconds of being hit by the initial laser pulse. That research showed that the transformation occurred much more rapidly than was previously believed. But the existence of debris from meteor impacts that is composed entirely of shocked glass suggests that stishovite may be a short-lived phase that can convert permanently to shocked glass after impact.
Overturning Assumptions
In the latest study, the scientists took advantage of the Matter in Extreme Conditions instrument at LCLS to generate shock waves that induced various peak pressures in silica samples. After sending the laser pulse, “We just watch what the silica does naturally,” said Gleason, who is the LANL Fredrick Reines Postdoctoral Fellow.
Analysis of X-ray diffraction data taken at various intervals after peak pressure was reached showed that when the pressure is high enough, stishovite forms, but it then reverts to shocked glass. The diffraction data from the LCLS samples matched data from impact debris collected in the field.
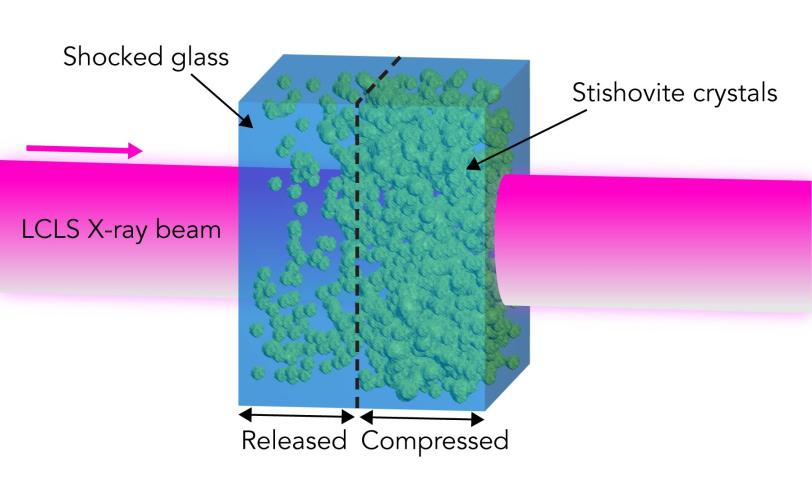
Scientists have previously assumed that peak pressures of roughly 40 gigapascals – equivalent to 400,000 times the atmospheric pressure around us – are required to create shocked glass from silica. But the results from this study suggest that the threshold is about 25 percent lower than that, and that stishovite then reverts to the shocked glass state due to thermal instability rather than higher pressure.
“An impact event has a short timeline,” said Gleason, “making LCLS an ideal instrument for understanding the fundamental thermodynamics of glasses formed by impacts.” Gleason envisions using the MEC at LCLS to investigate other Earth-abundant minerals, such as feldspar, and to better understand the “rule book” for transformation processes.
Gleason’s research is more broadly applicable to debris from other planets, such as meteorites from Mars that also contain shocked glass. Martian meteorites often contain trapped volatile compounds, such as water vapor and methane. No one understands how these compounds become locked inside meteorites or why they don’t escape, but continued work at LCLS could provide answers.
In addition to LANL and SLAC, researchers contributing to this study came from the Stanford Institute for Materials and Energy Sciences (SIMES), the DOE’s Lawrence Livermore National Laboratory, the Center for High Pressure Science and Technology Advanced Research in Shanghai, the Carnegie Institution of Washington’s High Pressure Synergetic Consortium, Friedrich Schiller University Jena in Germany and Stanford University. Major funding came from the DOE Office of Science and the National Science Foundation. Part of the work was carried out at the Advanced Light Source, a DOE Office of Science User Facility at DOE’s Lawrence Berkeley National Laboratory.
Citation: A.E. Gleason et al., Nature Communications, 14 November 2017 (10.1038/s41467-017-01791-y)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.





