Shedding Light
In 1971, physicist Burton Richter of Stanford Linear Accelerator Center was building a new type of particle collider called a storage ring.
By Lori Ann White
In 1971, physicist Burton Richter of Stanford Linear Accelerator Center was building a new type of particle collider called a storage ring. The lab’s two-mile-long linear accelerator—housed in what was then the longest building in the world—would shoot electrons and their antimatter twins, called positrons, into the 80-meter-diameter Stanford Positron Electron Accelerating Ring, and SPEAR would set the beams of particles on a collision course. Richter and his colleagues stood by to examine the debris to see what discoveries came out.
Physicists were already familiar with one product of accelerating particles in a circle at close to the speed of light: synchrotron radiation. To high-energy physicists, synchrotron energy was an annoyance, an unwanted demonstration of the laws of physics— in this case, a law that says charged particles radiate energy when accelerated. Synchrotron radiation would rob electrons and positrons in the SPEAR ring of energy that would better serve science by going into the creation of new particles and the illumination of new physics.
Two Stanford faculty members, condensed-matter physicist Sebastian Doniach and electrical engineer William Spicer, had a different vision. They saw in the SPEAR storage ring a new and better way to satisfy an already huge demand for X-rays. They saw a source of high-energy electromagnetic waves whose short wavelengths and highly penetrating nature could illuminate materials on a scale small enough to show atomic structures and reveal electronic properties.
Doniach and Spicer approached Richter even before SPEAR was finished.
“They said that they would revolutionize condensed-matter physics if only I would let the X-ray beam out of the storage ring. That was their pitch,” Richter recalls.
After a quick, informal inquiry to see if the two professors were “blowing smoke,” as he puts it— and obtaining assurances that modifying the ring would not endanger its high-energy physics mission— Richter said, “Let’s do it.”
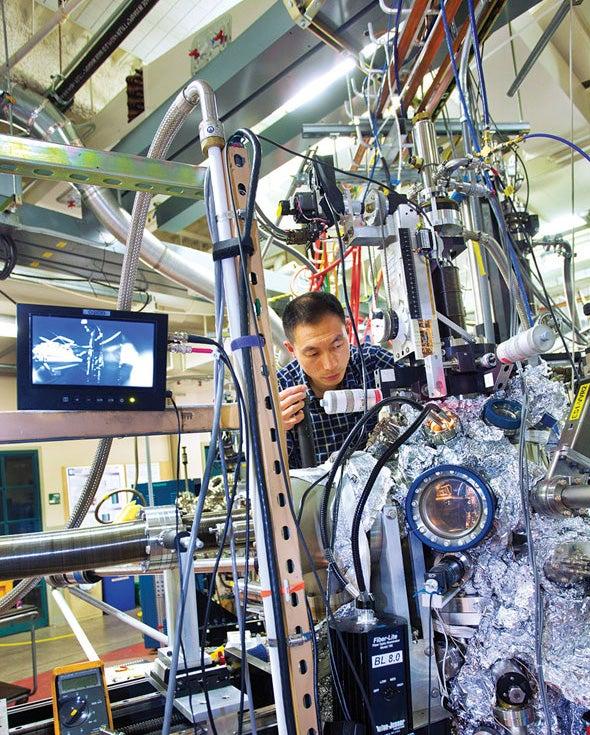
The SPEAR construction team removed a section of the ring and modified it, adding a beam port to “let the X-rays out.” And Richter did Doniach and Spicer another favor: “I bought them a Sears garden shed to use for experiments.”
The Stanford Synchrotron Radiation Project was under way.
As promised, the stronger, more intense X-rays streaming into the garden shed began making their mark on condensed-matter research and structural biology. Stunningly clear data soon proved the value of synchrotron radiation for investigating the chemical compositions and electronic structures of materials, while the first images of protein structures using such strong X-rays soon followed. Both breakthroughs came as Richter continued to smash together electrons and positrons; the X-ray experiments may have been parasitic on the main program, but the parasites did not disturb their host.
In 1974, Richter and his team discovered the J/psi particle, for which he shared the 1976 Nobel Prize in Physics.
Today, more than 70 light sources are in operation or under construction around the world, including the descendent of that original project, the Stanford Synchrotron Radiation Lightsource or SSRL.
No longer an afterthought, dedicated light sources are critical research tools for everything from developing better batteries to finding the causes of disease. They contribute to the development of new medicines. They delve into the structures of proteins, the secrets of photosynthesis, the behavior of catalysts. Developing high-performance materials, investigating pollution—any time a researcher wants to spy on a process or unravel a structure, if it’s on a molecular—or now atomic—scale, a light source is the tool of choice.
As Richter points out, “Doniach and Spicer were simply too modest when they said they were going to revolutionize condensed-matter physics. They revolutionized more than that. Having a million times more intense X-rays gave you capabilities that nobody ever dreamed of being able to exploit.”
Illuminating proteins
Those million-times-more-intense X-rays have transformed the study of structural biology, enabling scientists to get the first good look at the intricate cellular machines that build the structures of our bodies and generate the energy that powers us. True, researchers had deciphered some protein structures as far back as the late 1950s—long before the widespread use of light sources. However, by 1973, two years after being established and just as SPEAR was coming on line, the Protein Data Bank held just nine structures. Today the Protein Data Bank, a key resource for structural biologists the world over, holds the structures of more than 72,000 proteins.
The revolution did not happen overnight. At first, the primitive state of imaging technology made high-resolution results very hard to get. The protein crystals themselves were the biggest stumbling block.
As Bob Sweet, a beamline scientist at Brookhaven National Laboratory’s National Synchrotron Light Source, puts it: “You have to understand that protein crystals are the texture of firm Jell-O and very moist. They don’t do very well in an X-ray beam.”
Two breakthroughs—freezing the crystals to retard radiation damage, and the development of better detectors using CCD chips—transformed the use of synchrotron light sources, according to Thomas Steitz of Yale University, who shared the 2009 Nobel Prize in Chemistry for unraveling the structure of the ribosome, the cellular factory that builds proteins from genetic instructions brought by messenger RNA.
“It would have been impossible to have solved the ribosome without a synchrotron—period,” Steitz says. “In my opinion it’s a good thing there are light sources scattered everywhere.”
Discovering drugs
Academic crystallographers such as Steitz generally focus on determining the structures of biologically important proteins. Once those structures are unraveled, crystallographers from pharmaceutical companies home in on receptor sites on the proteins where disease-causing agents can latch on and wreak havoc. That’s the best place to ambush and disable them.
The techniques for studying these receptors are simpler to execute and faster to complete than determining the structure of an entire protein from scratch. But finding the right drug can still take years. Continually competing for access to beamlines at national facilities just won’t work for pharmaceutical companies—not if they want to get anything accomplished— yet light sources are generally national facilities for a reason. They’re big, complicated, and full of sophisticated machinery requiring highly specialized knowledge to maintain and operate—in other words, expensive. Very expensive.
Thus was born the Industrial Macromolecular Crystallography Association, or IMCA, a consortium of pharmaceutical companies that approached Argonne National Laboratory during the construction of the Advanced Photon Source. IMCA members would provide the money to build, equip, staff, and run beamlines; Argonne would provide the X-rays. The result is Sector 17 at the APS, two specialized beamlines dedicated to the fast turnaround of candidate substances for drugs.
“It’s rather remarkable that all the companies manage to cooperate,” says Lisa Keefe, director of the IMCA Collaborative Access Team program at the APS. “They are all competitors.” One reason the collaboration works: their work remains proprietary. Membership varies, but currently consists of Abbott, Bristol-Myers Squibb, Merck, Novartis and Pfizer, with subscription programs available to companies that are not IMCA members. As part of the deal with Argonne and the Department of Energy, the beamlines are also open to general users 25 percent of the time, with “general” referring to anyone who comes up with a good proposal. In practical terms, this means academic research groups.
Time is money to these companies, and their money has bought them time. According to Keefe, the beamlines are focused on very-high-throughput screening and data collection. With the state-of-the-art instrumentation at beamline 17-ID, researchers can collect a data set every three to five minutes.
One of the first members of IMCA, pharmaceutical giant Abbott Laboratories, used the APS to study HIV, the virus that causes AIDS. That research directly contributed to the development of Kaletra, a highly effective protease inhibitor that stops the HIV virus from replicating. GlaxoSmithKline relied on research conducted at the APS to create Votrient, a treatment for kidney cancer, and Merck developed Januvia, a treatment for diabetes.
To read the full article visit our symmetry website.