In brief: Watching molecules split in real time
A new method could be used to look at chemical reactions that other techniques can’t catch, for instance in catalysis, photovoltaics, peptide and combustion research.
Using a new X-ray technique, a team of researchers was able to watch in real time as a molecule split apart into two new molecules. The method could be used to look at chemical reactions that other techniques can’t catch, for instance in catalysis, photovoltaics, peptide and combustion research. The team, led by researchers from Brown University in collaboration with the Department of Energy’s SLAC National Accelerator Laboratory, published their results in March in Angewandte Chemie.
The molecule, trimethylamine, comprises a nitrogen with 3 methyl groups on it. At SLAC’s Linac Coherent Light Source (LCLS) X-ray laser, the researchers used X-ray scattering to measure changes in the structure of the molecule and how its electrons are arranged. They watched as one of the methyl groups split off when the molecule was excited with light and found that while some of these methyls split off quickly, in about 640 millionths of a billionth of a second, others took their time, splitting off about 100 times slower.
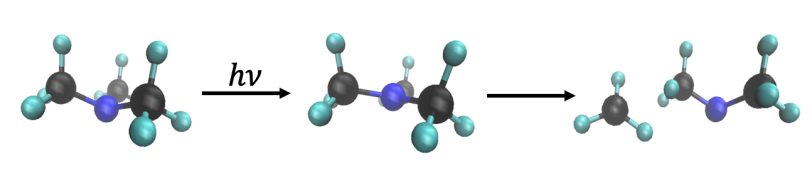
What sets this study apart from others is that the researchers measured the process over a wide range of timescales. They also managed to reduce background noise, which, together with the brightness of LCLS, enabled them to catch signals they might otherwise have missed.
Jennifer Ruddock, a doctoral candidate at Brown University, was the study’s lead author. SLAC’s Mike Minitti and Brown’s Peter Weber were the principal investigators. Researchers from the University of Edinburgh in the UK compared the data to theory. LCLS is a DOE Office of Science user facility. This work was largely funded by the DOE Office of Science.
Citation: Jennifer Ruddock et al., Angewandte Chemie, 13 March 2019 (10.1002/anie.201902228)
Contact
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.