Scientists Make the First Molecular Movie of One of Nature’s Most Widely Used Light Sensors
The X-ray laser movie shows what happens when light hits retinal, a key part of vision in animals and photosynthesis in microbes. The action takes place in a trillionth of an eye blink.
By Glennda Chui
Scientists have made the first molecular movie of the instant when light hits a sensor that's widely used in nature for probing the environment and harvesting energy from light. The sensor, a form of vitamin A known as retinal, is central to a number of important light-driven processes in people, animals, microbes and algae, including human vision and some forms of photosynthesis, and the movie shows it changing shape in a trillionth of an eye blink.
“To my knowledge, nobody has measured changes in a retinal biosensor so quickly and so accurately,” said Jörg Standfuss, a biologist at the Paul Scherrer Institute (PSI) in Switzerland who led the research at the Department of Energy’s SLAC National Accelerator Laboratory. “And the fact that we saw just the opposite of what we intuitively expected was spectacular and surprising to us.”
The team carried out their experiments at the lab’s Linac Coherent Light Source (LCLS) X-ray laser and reported the results today in Science.
In the past, scientists had to fill the gaps in their knowledge about retinal’s behavior by making inferences based on theory and computer simulations, said Mark Hunter, a staff scientist at LCLS and paper co-author. But in this study, “LCLS’s super-short pulses allowed us to collect data on where the atoms actually were in space and how that changed over time,” he said, “so it gave us a much more direct visualization of molecules in motion.”
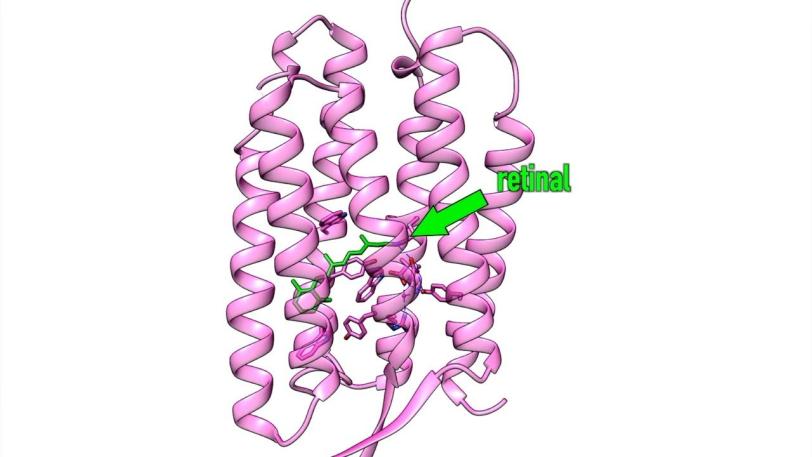
LCLS CXI | The First Molecular Movie of One of Nature’s Most Widely Used Light Sensors
Colorful Lakes and Arching Cats
Retinal is so central to human vision – it’s named for the retina at the back of the eye – that scientists have been studying it for nearly a century, steadily building a more detailed picture of how it works. It’s also used in the burgeoning field of optogenetics to turn groups of nerve cells on and off, revealing how the brain works and how things go wrong in conditions like depression, stroke and addiction.
The retinal studied in this experiment came from salt-loving microbes that use it to harvest energy from the sun. (Fun fact: Purple and orange-red pigments in these microbes give the briny waters they live in, from San Francisco Bay salt ponds to Senegal’s Lake Retba, their incredibly vivid colors.)
Retinal does its job while snuggled deep into a pocket of specialized proteins in the membrane of the cell. When hit by light, the retinal changes shape – in this case it curves, like a cat arching its back. This creates a signal that’s transmitted by the protein into the cell’s interior, initiating photosynthesis or vision.
Scientists thought retinal set off the signal by pushing on the protein pocket as it changed shape. But the LCLS experiments found just the opposite: The pocket actually changed shape first, creating space for the retinal to perform its arching-cat maneuver. Nearby water molecules also moved aside and made room, Standfuss said. It all took place within 200 to 500 femtoseconds, or millionths of a billionth of a second. That’s about a trillionth of the blink of an eye, making this one of the fastest chemical reactions known in living things.
“In retrospect, this makes a lot of sense,” Standfuss said. “We always say seeing is believing in structural biology, and in this case it’s very true. The molecular movie we made makes it so obvious what’s going on that you can immediately grasp it. This solves a very important piece of the puzzle of how retinal works that people have been wondering about.”
The protein pocket’s initial movements are triggered by small changes in electrical charge that rearrange certain chemical bonds, he said. These movements guide the retinal’s response and make it much more efficient, which is why it requires only a few photons of light and why nature can use that light so effectively.
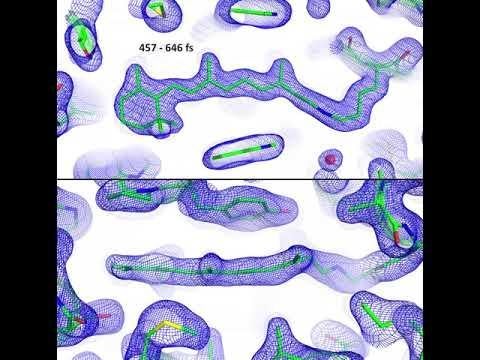
LCLS | Molecular Movie of Retinal
Catching Molecules in Action
How can you watch something so small that happens so fast? The X-ray laser was key, Standfuss said. LCLS produces brilliant pulses of X-ray laser light that scatter off the electrons in a sample and reveal how its atoms are arranged. Like a camera with an extreme zoom lens and ultrafast shutter speed, the X-ray laser can also make snapshots of molecules moving, breaking apart and interacting with each other.
In this case, the researchers looked at samples of retinal snuggled into pockets of bacteriorhodopsin, a purple protein found in simple microbes like those in the salt ponds.
After years of effort, PSI postdoctoral researcher Przemyslaw Nogly, the lead author of the report, found ways to pack these retinal-protein pairs into thousands and thousands of tiny but well-ordered crystals. One after another, crystals were hit with light from an optical laser – a stand-in for sunlight – followed by X-ray laser pulses to record the response. Then Nogly and the team boiled down data into 20 snapshots and assembled them into stop-action movies that show the retinal moving in sync with its protein pocket.
Proteins like bacteriorhodopsin that sit in cell membranes are notoriously difficult to study because it’s so hard to form them into crystals for X-ray experiments, Hunter said. But scientists have learned that they crystallize more readily when embedded in a fatty, toothpaste-like sludge that mimics their natural environment, and that’s how these crystals were formed and delivered into the X-ray beam.
The researchers were also able to detect “protein quakes,” vibrations that release some of the energy deposited by the light flashes. These had been predicted by theory and came off as expected.
Standfuss said he has spent most of his career studying retinal and its role in vision, which involves slightly different shape changes in the protein-embedded molecule. “I really hope that we can now study the same reaction in many different systems,” he said. “Now that we see for the first time how it works in one particular bacterial protein, I want to understand how it works in the human eye as well.”
LCLS is a DOE Office of Science user facility, and LCLS researchers Sergio Carbajo, Jason Koglin, Matthew Seaberg and Thomas Lane were co-authors of this study. Other contributors came from PSI, the University of Gothenburg in Sweden, the Fritz Haber Center for Molecular Dynamics at the Hebrew University of Jerusalem, the RIKEN SPring-8 Center and Kyoto University in Japan, the Center for Free-Electron Laser Science at DESY in Germany and Arizona State University. Major funding came from the European Horizon 2020 Program, the Swedish Research Council and the Swiss National Science Foundation.
Citation: P. Nogly et al., Science (10.1126/science.aat0094)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.





