X-ray Laser Gets First Real-time Snapshots of a Chemical Flipping a Biological Switch
Scientists used SLAC's LCLS X-ray laser to make the first snapshots of a chemical interaction between two biomolecules. It changes the shape of millions of molecular switches almost instantaneously, like synchronized swimmers performing the same move.
Menlo Park, Calif. — Scientists have used the powerful X-ray laser at the Department of Energy’s SLAC National Accelerator Laboratory to make the first snapshots of a chemical interaction between two biomolecules – one that flips an RNA “switch” that regulates production of proteins, the workhorse molecules of life.
The results, published today in Nature, show the game-changing potential of X-ray free-electron lasers, or XFELs, for studying RNA, which guides protein manufacturing in the cell, serves as the primary genetic material in retroviruses such as HIV and also plays a role in most forms of cancer.
CXI | X-ray Laser Gets First Real-time Snapshots of a Chemical Flipping a Biological Switch
In a landmark experiment at SLAC National Accelerator Laboratory, scientists used an X-ray laser to capture the first snapshots of a chemical interaction between two biomolecules in real time and at an atomic level.
SLAC National Accelerator Laboratory
And because this particular type of RNA switch, known as a riboswitch, is found only in bacteria, a deeper understanding of its function may offer a way to turn off protein production and kill harmful germs without causing side effects in the humans they infect.
“Previous experiments at SLAC’s X-ray laser have studied biological reactions like photosynthesis that are triggered by light. But this is the first to observe one that is triggered by the chemical interaction of two biomolecules in real time and at the atomic scale,” said Yun-Xing Wang, a structural biologist at the National Cancer Institute’s Center for Cancer Research who led the international research team.
“This really demonstrates the unique capability that X-ray free-electron lasers offer that no current technology, or any other technology on the horizon, can do. It’s like you have a camera with a very fast shutter speed, so you can catch every move of the biomolecules in action.”
The experiments were carried out at SLAC’s Linac Coherent Light Source (LCLS), a DOE Office of Science User Facility. They are the first to demonstrate how XFELs can take snapshots and potentially make movies of RNA and other biomolecules as they chemically interact – offering glimpses into fundamental workings of the cell that can’t be obtained any other way.
Seeing RNA Shape Shifting
RNA is a key part of the genetic material in all living cells. It comes in several types that work together to guide the production of proteins by the cell’s ribosomes, according to blueprints encoded in DNA.
But both DNA and RNA also contain extensive regions that don’t code for any protein – the so-called genetic “dark matter.” Scientists thought for many years that these regions didn’t do anything. Now they know that they play an important role in determining where and when genes turn on and off and otherwise fine-tuning their function. The vast majority of cancers are due to mutations in these non-coding regions, Wang said, so understanding how these regions work is important for cancer research as well as fundamental biology.
However, figuring out what the RNA non-coding regions do is difficult. RNA molecules are wobbly and flexible, so it’s hard to incorporate them into the large crystals typically needed to study their atomic structure at X-ray light sources.
LCLS removes this barrier by allowing scientists to get structural information from much smaller, nanosized crystals, which are much easier to make. Its powerful X-ray laser pulses, a billion times brighter than any available before, are so short that they collect data from each crystal in a few millionths of a billionth of a second, before damage from the X-rays sets in.
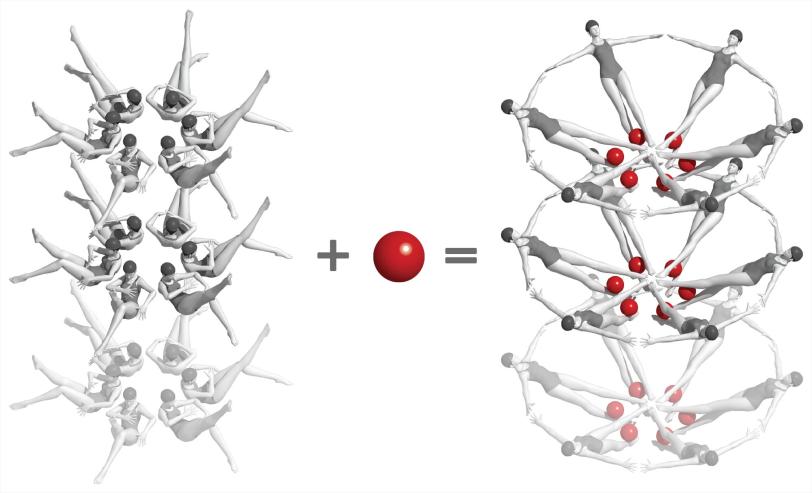
Wang’s team studied a riboswitch from Vibrio vulnificus, a bacterium related to the one that causes cholera. The riboswitch sits in a long strand of messenger RNA (mRNA), which copies DNA’s instructions for making a protein so they can be read and carried out by the ribosome. The switch acts like a thermostat that regulates protein production.
In this case, the mRNA guides production of a protein that in turn helps to produce a small molecule called adenine. When there is too much adenine in the bacterial cell, adenine molecules enter pockets in the riboswitches and flip the riboswitches into a different shape, and this changes the pace of protein and adenine production.
First Stills of an Elegant Film
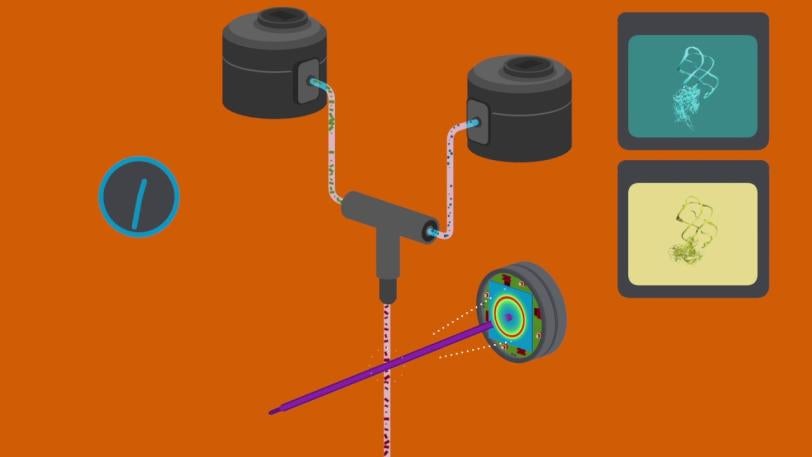
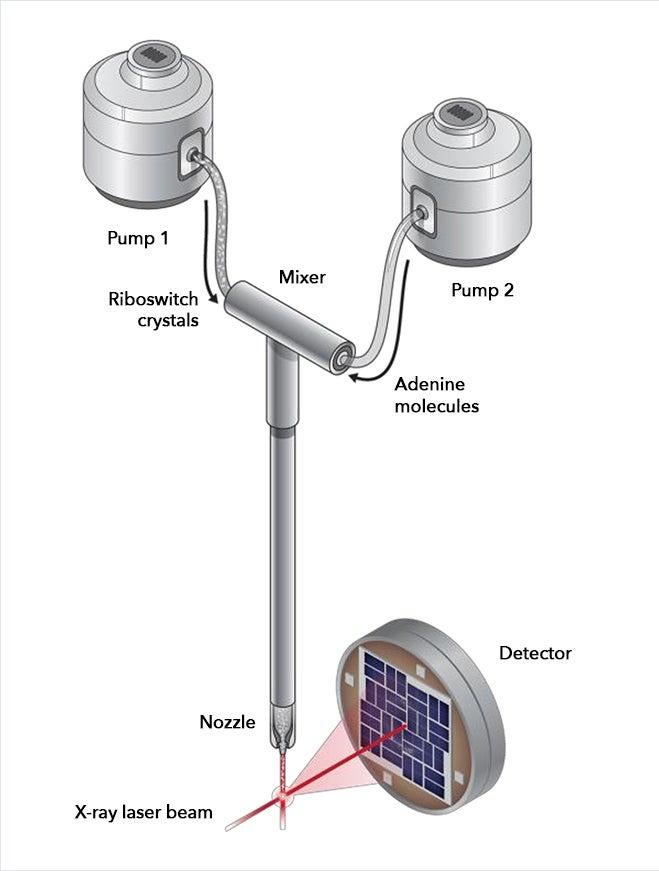
For the LCLS experiments, the researchers made nanocrystals that incorporated millions of copies of the riboswitch and mixed them with a solution containing adenine molecules. Each crystal was so small that adenine could quickly and uniformly penetrate into every corner of it, enter riboswitch pockets and flip them almost instantaneously, as if they were millions of synchronized swimmers executing a single flawless move.
The scientists took snapshots of this interaction by hitting the crystals with X-ray laser pulses at carefully timed intervals after the mixing started. This gave them the first glimpse of a fleeting intermediate stage in the process, which occurred 10 seconds in. Separately, they obtained the first images of the riboswitch in its initial, empty-pocket state, and discovered that it existed in two slightly different configurations, only one of which participates in switching.
The researchers were surprised to discover that the sudden change in the shape of the riboswitches was so dramatic that it changed the shape of the entire crystal, too. Normally a major change like this would crack the crystal and spoil the experiment. But because these crystals were so small they held together, so the X-ray laser could still get structural information from them.
“To me it’s still a mystery how the crystal managed to do that,” said Soichi Wakatsuki, a professor at SLAC and at the Stanford School of Medicine and head of the lab’s Biosciences Division, who was not part of the research team. “This actually opens up a lot of new possibilities and gives us a new way to look at how RNA and proteins interact with small molecules, so this is very exciting.”
In addition to the National Cancer Institute and SLAC’s LCLS, scientists contributing to the research came from Arizona State University, Johns Hopkins University, the Center for Free-Electron Laser Science at Deutsches Elektronen-Synchrotron (DESY), University of Hamburg, Hauptmann-Woodward Medical Research Institute, the National Institutes of Health and the DOE’s Argonne National Laboratory.
Funding came from the National Science Foundation and the NIH Intramural Research Programs.
Citation: J.R. Stagno et al., Nature, 14 November 2016 (10.1038/nature20599)
Press Office Contact: Manuel Gnida, mgnida@slac.stanford.edu, (650) 926-2632
About SLAC
SLAC National Accelerator Laboratory explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by researchers around the globe. As world leaders in ultrafast science and bold explorers of the physics of the universe, we forge new ground in understanding our origins and building a healthier and more sustainable future. Our discovery and innovation help develop new materials and chemical processes and open unprecedented views of the cosmos and life’s most delicate machinery. Building on more than 60 years of visionary research, we help shape the future by advancing areas such as quantum technology, scientific computing and the development of next-generation accelerators.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.