Batteries come in many shapes and sizes, but their materials can be hard to source. SLAC researchers are trying to build them with more abundant and ethically mined elements.
By David Krause
Over the past decade, SLAC scientist Johanna Nelson Weker has watched hundreds of batteries charge, discharge, and ultimately fizzle out. Her team’s research has improved the understanding of battery reliability. More reliable batteries are needed to power more things than ever, including electric grid storage facilities, cars, trucks and even leaf blowers.
But the story of battery proliferation can’t remain focused on performance first, Nelson Weker says. The industry has arrived at its next big fork in the road: how to build batteries using abundant and ethically mined materials. The most popular type of batteries today, lithium ion, requires materials that can be hard to source – and some of which have documented human rights concerns associated with mining practices outside of the United States.
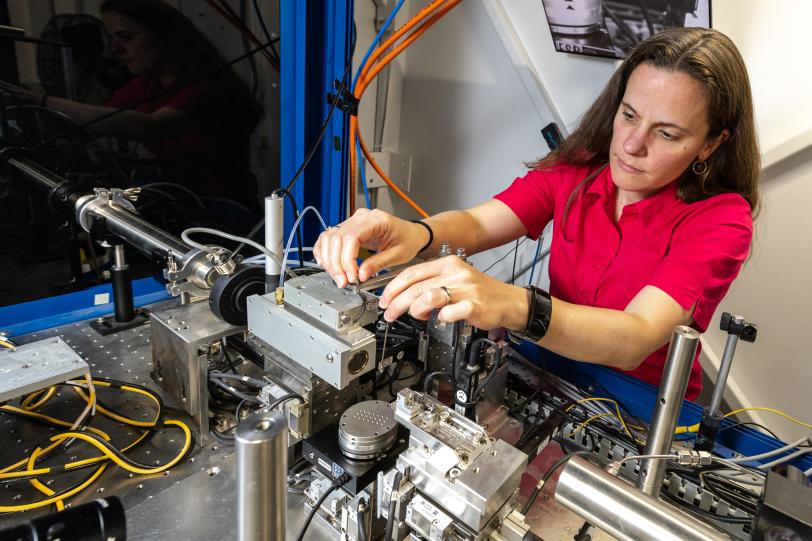
Nelson Weker’s team at the Department of Energy’s SLAC National Accelerator Laboratory is currently focused on finding ways to design batteries that use alternative materials, like zinc and manganese, rather than lithium and cobalt. They do this at the lab's Stanford Synchrotron Radiation Lightsource (SSRL), specifically by zapping battery material samples with the facility’s bright, powerful X-rays. The X-rays allow the researchers to understand a battery’s atomic behavior as it charges and discharges electricity.
In this Q&A, Nelson Weker tells us what the future of battery design could look like.
What’s the urgency behind designing more sustainable batteries?
We’ve gotten to the point where we know how to make good batteries that perform well for many applications, like driving an electric vehicle. So there is less of a need to prove that batteries are able to keep up in terms of performance with other energy resources.
Now we want to build them in an even more sustainable way, such as by using abundant materials, rather than lithium and cobalt, which are more limited resources.
Additionally, consumers are becoming educated about the environmental impact of building something like an electric vehicle and they want to help reduce waste, increase recyclability, and remove toxic chemicals from batteries. That’s where using more abundant battery materials, like zinc and manganese, becomes critical. These can reduce the negative impacts of mining materials.
What battery research projects are you’re working on?
We have a few primary projects, the first of which involves finding ways to reduce the amount of cobalt in EV batteries. Cobalt is a difficult resource to mine and there are sociopolitical issues associated with mining it. We want to develop an EV battery that needs little to no cobalt, but that can still fast-charge like a normal EV battery. People want fast-charging EVs, so maintaining this ability is critical.
A second project is how to use disordered rock salts in a battery’s cathode or positive terminal. Disordered rock salts (DRX) have a more randomized atomic structure than traditional cathodes and could reduce or eliminate the need for cobalt and nickel in the cathode. We are working with Lawrence Berkeley National Laboratory, and our first problem to solve is why only certain DRXs work as cathodes. Here at SLAC, we plan to use SSRL to study the chemical makeup of DRX structures to understand the synthesis process to the final product. Sometimes you try and synthesize a material like DRX and you don’t get the ratio of different elements that you were aiming for.
We are also looking at how to design a battery that uses zinc or sulfur. Sulfur is a super earth abundant element. It’s actually a byproduct of the petroleum industry, where it’s considered waste.
Could these alternative battery designs also improve performance?
Not necessarily, and that’s okay. We think that performance shouldn’t be the only key design variable if we want batteries to become more usable throughout society.
Our main goal is therefore to design batteries that perform the same as the popular lithium-ion batteries, but with materials that are widely available and more ethically mined.
What other alternative materials are you looking into?
Zinc is one of my favorites because it uses a multivalent ion, meaning it can provide two electrons during a battery’s discharging process, while lithium provides only one electron. Zinc also works with water-based electrolytes, which reduces fire hazards associated with batteries. This makes it a great option for large energy storage facilities for the grid.
The tradeoff is that zinc is heavier than lithium, meaning it would increase the overall weight of something like an EV. There is a lot of room for improvement with zinc batteries, similar to the amount of room for improvement we had with lithium-ion batteries 20 years ago. Zinc batteries are also recycled more often than lithium batteries. That’s a nice additional thing – there is already a mechanism in place to recycle zinc batteries.
How does X-ray science at SSRL help your team study batteries?
At SSRL, we can study batteries' atomic structures and the movement of their ions and electrons while we operate them. This allows us to watch batteries perform over many cycles and determine what causes them to deteriorate and fail.
We can also look at the synthesis of the battery components – all the intermediate chemical phases that occur in battery reactions. Mapping intermediate steps provides a deeper understanding of a how the synthesis of a battery material could be improved.
Do you have a favorite battery discovery at SSRL?
This is a tricky question since research tends to be a series of small victories rather than distinct moments. I guess one moment was when we confirmed that we could use X-ray diffraction to map lithium metal plating – which can cause batteries to malfunction – without needing to disassemble them. Normally to confirm lithium plating, scientists disassemble the batteries to visually inspect them.
What are you most excited to see in battery research over the next five years?
Getting more abundant cathode materials. It’s important that we are not just trying to chase more capacity and performance. Again, we’re trying to think about all of the other things that matter when designing a better battery, or anything for that matter.
I’m also excited to see the focus of the industry shift toward an emphasis on stationary battery storage technology. Historically, lithium-ion battery research has been pushed by consumer electronics. Then in the last 10 years, it was pushed by EVs. Now is finally the time when the push isn’t coming from those two things. It’s going to come from the need for long-duration energy storage.
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
About SLAC
SLAC National Accelerator Laboratory explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by researchers around the globe. As world leaders in ultrafast science and bold explorers of the physics of the universe, we forge new ground in understanding our origins and building a healthier and more sustainable future. Our discovery and innovation help develop new materials and chemical processes and open unprecedented views of the cosmos and life’s most delicate machinery. Building on more than 60 years of visionary research, we help shape the future by advancing areas such as quantum technology, scientific computing and the development of next-generation accelerators.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.







