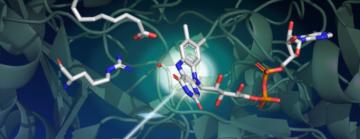
Scientists uncover surprising behavior of a fatty acid enzyme with potential biofuel applications
Derived from microscopic algae, the rare, light-driven enzyme converts fatty acids into starting ingredients for solvents and fuels.
By Jennifer Huber
Although many organisms capture and respond to sunlight, it’s rare to find enzymes – proteins that promote chemical reactions in living things – that are driven by light. Scientists have identified only three so far. The newest one, discovered in 2017, is called fatty acid photodecarboxylase (FAP). Derived from microscopic algae, FAP uses blue light to convert fatty acids into hydrocarbons that are similar to those found in crude oil.
“A growing number of researchers envision using FAPs for green chemistry applications because they can efficiently produce important components of solvents and fuels, including gasoline and jet fuels.” says Martin Weik, the leader of a research group at the Institut de Biologie Structurale at the Université Grenoble Alpes.
Weik is one of the primary investigators in a new study that has captured the complex sequence of structural changes, or photocycle, that FAP undergoes in response to light, which drives this fatty acid transformation. Researchers had proposed a possible FAP photocycle, but the fundamental mechanism was not understood, partly because the process is so fast that it’s very difficult to measure. Specifically, scientists didn't know how long it took FAP to split a fatty acid and release a hydrocarbon molecule.
Experiments at the Linac Coherent Light Source (LCLS) at the Department of Energy’s SLAC National Accelerator Laboratory helped answer many of these outstanding questions. The researchers described their results in Science.
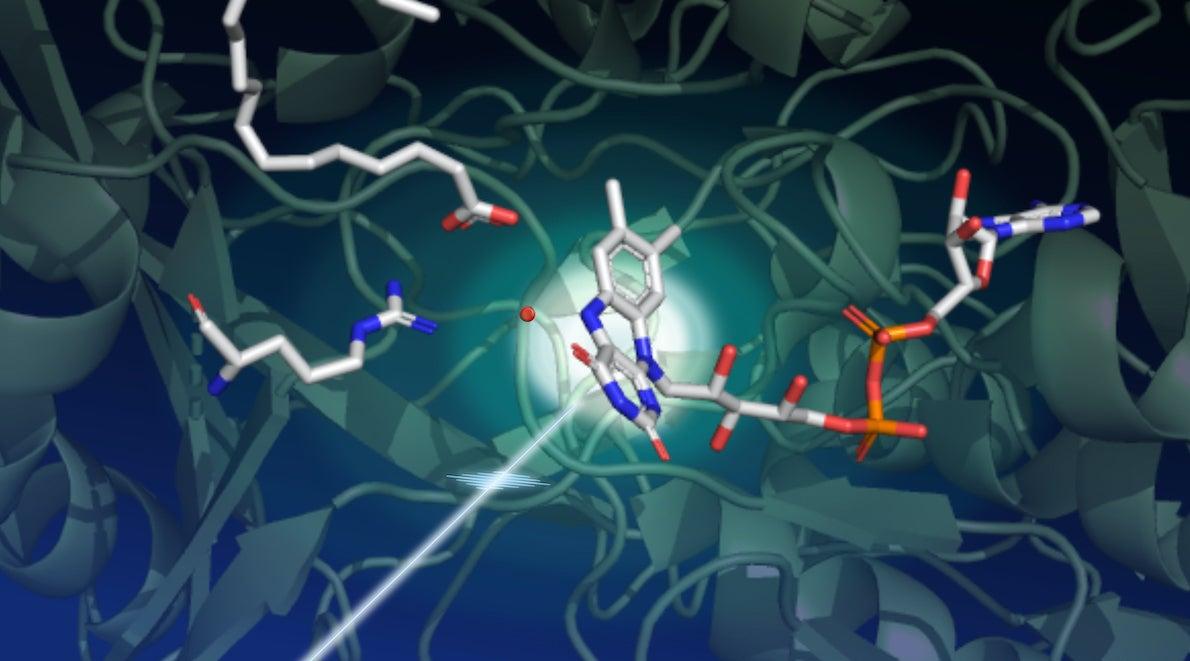
All the tools in a toolbox
To understand a light-sensitive enzyme like FAP, scientists use many different techniques to study processes that take place over a broad range of time scales. For instance, photon absorption happens in femtoseconds, or millionths of a billionth of a second, while biological responses on the molecular level often happen in thousandths of a second.
“Our international, interdisciplinary consortium, led by Frédéric Beisson at the Université Aix-Marseille, used a wealth of techniques, including spectroscopy, crystallography and computational approaches,” Weik says. “It’s the sum of these different results that enabled us to get a first glimpse of how this unique enzyme works as a function of time and in space.”
The consortium first studied the complex steps of the catalytic process at their home labs using optical spectroscopy methods, which investigate the electronic and geometric structure of atoms in the samples, including chemical bonding and charge. Spectroscopic experiments identified the intermediate states of the enzyme that accompanied each step, measured their lifetimes and provided information on their chemical nature. These results revealed the need for the ultrafast capabilities of the LCLS X-ray free-electron laser (XFEL), which can track the molecular motion with atomic precision.
A structural view of changes in the FAP molecule during the catalytic process was provided by serial femtosecond crystallography (SFX) at the LCLS. During these experiments, a jet of tiny FAP microcrystals was hit with optical laser pulses to kick off the catalytic reaction. This ensured that all the molecules react at a similar time, synchronizing their behavior and making it possible to track the process in detail. Extremely brief, ultrabright X-ray pulses then measured the resulting changes in the enzyme’s structure.
By integrating thousands of these measurements – acquired using various time delays between the optical and X-ray pulses – the researchers were able to follow structural changes in the enzyme. They also determined the structure of the enzyme’s resting state by probing without the optical laser.
Surprisingly, the researchers found that in the resting state, the light-sensing part of the enzyme has a bent shape. “This small molecule, called the FAD cofactor, is a derivative of vitamin B2 that acts like an antenna to capture photons,” Weik says. “It absorbs blue light and initiates the catalytic process. We thought the starting point of the FAD cofactor was planar, so this bent configuration was unexpected.”
The bent shape of the FAD cofactor was first discovered by X-ray crystallography at the European Synchrotron Radiation Facility, but the scientists had suspected this bend was an artifact of radiation damage, a common problem for crystallographic data collected at synchrotron light sources.
“Only SFX experiments could confirm this unusual configuration because of their unique ability to capture structural information before damaging the sample,” Weik says. “These experiments were complemented by computations. Without the high-level quantum calculations performed by Tatiana Domratcheva of Moscow State University, we wouldn't have understood our experimental results.”
Next steps
Even with this improved understanding of FAP’s photocycle, unanswered questions remain. For example, researchers know carbon dioxide is formed during a certain step of the catalytic process at a specific time and location, but they don’t know if it is transformed into another molecule before leaving the enzyme.
“In future XFEL work, we want to identify the nature of the products and to take pictures of the process with a much smaller step size so as to resolve the process in much finer detail,” says Weik. “This is important for fundamental research, but it can also help scientists modify the enzyme to do a task for a specific application.”
Such precision experiments will be fully enabled by upcoming upgrades to the LCLS facility that will increase its pulse repetition rate from 120 pulses per second to 1 million pulses per second, transforming scientists’ ability to track complex processes like this.
Other researchers are already working towards industrial FAP applications, including a group that is designing an economic way to produce gases such as propane and butane.
The interdisciplinary consortium included researchers from the Institute of Structural Biology in Grenoble, Max Planck Institute for Medical Research in Heidelberg, Université Aix-Marseille, Ecole Polytechnique in Paris-Palaiseau, the Integrative Biology of the Cell Institute in Paris-Saclay, Moscow State University, the ESRF and SOLEIL synchrotrons in Grenoble and Paris-Saclay, and the team at SLAC National Accelerator Laboratory.
LCLS is a DOE Office of Science user facility. Major funding for this work came from the French National Research Agency (ANR).
Citation: D. Sorigué et al., Science, 9 April 2021 (10.1126/science.abd5687)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a vibrant multiprogram laboratory that explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by scientists around the globe. With research spanning particle physics, astrophysics and cosmology, materials, chemistry, bio- and energy sciences and scientific computing, we help solve real-world problems and advance the interests of the nation.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit energy.gov/science.