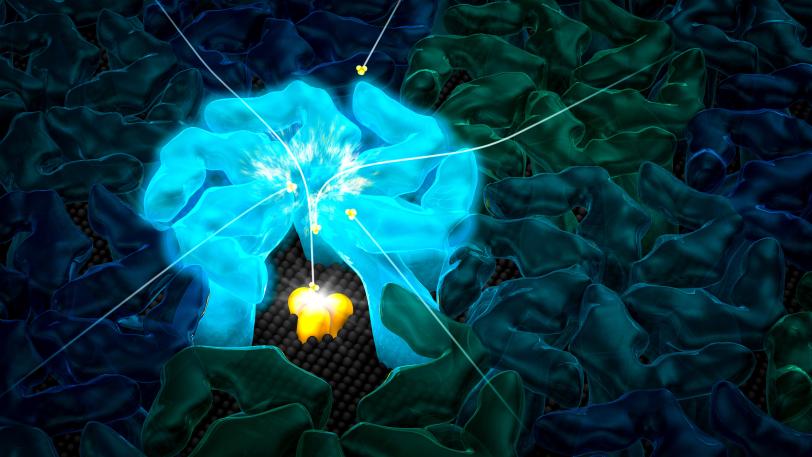
SLAC, Stanford Scientists Discover How a Hardy Microbe’s Crystalline Shell Helps it Reel in Food
Tiny pores in the shells of archaea microbes attract ammonium ions that are their sole source of energy, allowing them to thrive where this food is so scarce that scientists can’t even detect it.
By Glennda Chui
Microbes called archaea are found virtually everywhere on the planet. They play a major role in the vast global cycles that make carbon and nitrogen available to all living things. They’re also famous for living in hostile environments like the deep ocean where there’s way too little food for anything else to survive.
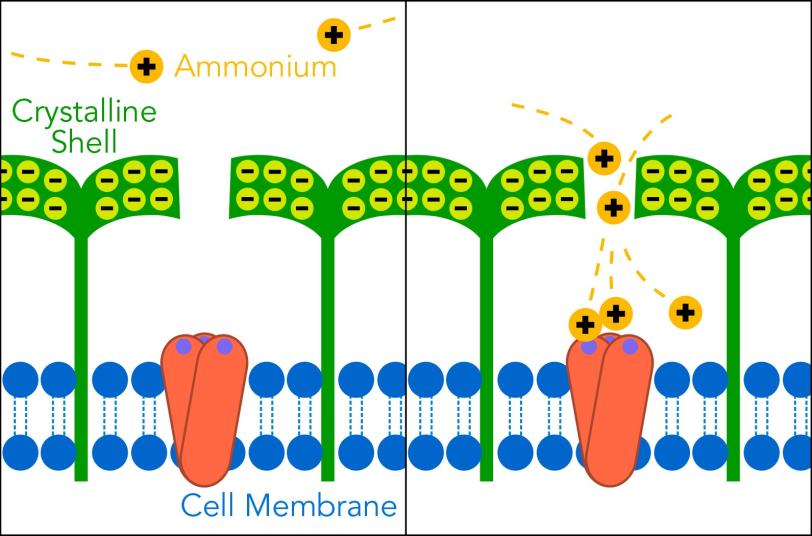
Now SLAC and Stanford scientists have discovered how some archaea thrive where other organisms would starve: Their crystalline shells not only protect them from the environment, but they also draw in nutrients through nanosized pores. Those nutrients concentrate in the space between the shell and the microbial cell, so what looks like a famine turns into a feast.
Their study, published today in the ISME Journal, has implications not just for understanding archaea, which make up one of the three major branches of the Tree of Life, but for using the mechanisms these tiny creatures have evolved to make inventions of our own.
“There is a lot of recent interest in naturally occurring nanochannels like the ones in archaeal shells,” said Henry van den Bedem, a senior staff scientist who is leading and developing a computational biology program at the Department of Energy’s SLAC National Accelerator Laboratory.
“They have all kinds of nice properties we could use in nanoscale design and engineering of things like sensors or battery technologies,” he said. “We know that nanochannels like the pores in these archaea are very selective about what they allow in or out. We can use computer simulations to study how they control this transport under many different conditions. It helps us understand how nature came up with these systems over billions of years so we can try to mimic that with synthetic nanochannels.”

Ammonia Eaters from the Mud
The archaea are an ancient and diverse group of microbes. This study focused on two species of archaea – one isolated from gravel at the bottom of the Seattle Aquarium, the other from mud at the bottom of San Francisco Bay.
Both species are ammonia-oxidizing archaea, or AOA, one of the most widespread groups of organisms on Earth, van den Bedem said. They get their energy from the most common form of ammonia, called ammonium. AOA can get by in places where the concentration of ammonium is just one-thousandth of the level that other ammonia-eating microbes require – so low that scientists can’t even measure it directly.
How do archaea do it? Van den Bedem led a highly interdisciplinary team to find out, teaming up with Professor Soichi Wakatsuki of SLAC and the Stanford School of Medicine and Stanford University Professor Christopher Francis, who has been studying AOA since their discovery a dozen years ago.
They thought the microbes’ crystalline shells with their tiny pores might play a role. Although the proteins that make up the shells vary a lot, they all have something in common: They carry high electric charges and arrange themselves in highly symmetric patterns. “Archaea invest a lot in making these highly charged crystalline layers around themselves. It must be an enormous burden,” van den Bedem said. “The fact that these features are so widespread suggests that they must be important for the organism’s survival.”
They’re Beautiful in Close-ups
To examine the shell and its pores in more detail, paper co-author Grant Jensen of the California Institute of Technology made images of them with cryo-electron tomography, a variation on electron microscopy. The images show that the proteins in the shell are arranged in “a beautiful hexagonal pattern,” as van den Bedem puts it. From the images, the researchers were able to measure the distances between the nanopores that appear to exist at the center of each hexagon.

Po-Nan Li, a Stanford graduate student and lead author of the study, used this information and other data to carry out computer simulations at the Stanford Research Computing Center to see how the shell’s architecture and electrical charge affect the microbe’s ability to get the food it needs.
The results suggest that the negatively charged nanopores attract ammonium, which has a positive charge. The ammonium travels through the pore into the space between the shell and the microbe. There it meets up with an enzyme complex embedded in the microbe’s outer membrane, which promotes a chemical reaction that liberates some of the ammonium’s electrons. Those electrons fuel the archaea’s activities, much as food fuels ours.
“Here’s a group of microbes that we didn’t even know existed until a decade or so ago, but they’re actually the primary organisms using ammonium as an energy source in many environments,” Francis said. “We’re somewhat limited in how we can study them because they’re tricky to cultivate. Often you get a culture going in the lab and then it dies. They’re very fussy and it’s challenging to mimic their natural environment, where they live with other organisms around them.”
Studies like this one, he said, open another window on these hardy microbes and the roles they play in the chemistry and life of our planet.
Next Step: A Much More Detailed Picture
This work brought together two independent research collaborations – one between Wakatsuki and David Stahl, a professor at the University of Washington, the other between Francis and John Bargar, a senior staff scientist at SLAC. Stanford PhD candidate Jonathan Herrmann and postdoctoral scholar Bradley Tolar played key roles in ensuring that the information in the computer model accurately reflects AOA physiology.
“This was a real interdisciplinary effort, bringing in computational biology, environmental genetics, biochemistry, and cell and structural biology to shed light on the fundamental question of how toxic ammonia is oxidized for energy and as part of the global nitrogen cycle,” Wakatsuki said. “The next step will be to try for much higher resolution images of these archaea and their shell structures with a new suite of cryo-EM instruments that recently became available on the SLAC campus.”
The research was funded by the DOE Office of Science and a DOE Laboratory Directed Research and Development program grant from SLAC.
Citation: P-N Li et al., ISME Journal, 13 June 2018 (10.1038/s41396-018-0191-0)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time.