New Program Broadens the Reach of LCLS Crystallography Experiments
A new screening program will allow researchers to quickly confirm whether precious biological samples yield useful information when struck by the intense X-ray pulses at SLAC's Linac Coherent Light Source (LCLS).
By Glenn Roberts Jr.
A new screening program will allow researchers to quickly confirm whether precious biological samples yield useful information when struck by the intense X-ray pulses at SLAC's Linac Coherent Light Source (LCLS).
Twenty-eight crystal screening slots are already scheduled for October 2013 through March 2014, and new proposals are now being sought for a batch of 20 screening shifts for the next LCLS run, which begins in April 2014. The deadline for those proposals is Oct. 22.The Protein Crystal Screening Program opens up multiple 6-hour shifts at LCLS to both newcomers and returnees. It has two goals: to broaden access to LCLS and to improve the success and productivity of X-ray crystallography experiments during regular runs of more extended experiments, which are in such high demand that fewer than one in four proposals can be accepted.
"This screening is potentially giving you an edge in telling you how to focus your time and effort to get the most out of an experiment," said SLAC's Marc Messerschmidt, an LCLS staff scientist who leads the upstart program.
Crystallizing biological molecules for study by the X-ray beam can be costly and time-intensive, and the screening shifts are an effort to accommodate scientific teams that may have only small amounts of sample to work with or may not have all the needed experimental tools.
LCLS is an ideal place for such experiments because it allows scientists to study very small sample crystals – "nanocrystals" – that are too small for study at other X-ray facilities.
Messerschmidt led a test run for the screening program in March 2013 using the Coherent X-ray Imaging (CXI) end station at LCLS, which is specially designed for experiments on small biological samples such as nanocrystals. Seven groups participated in that demonstration, which provided valuable data and insight, Messerschmidt said.
The groups all used a standardized setup for X-ray nanocrystallography, in which X-ray pulses strike and diffract through the tiny crystals, producing patterns in a detector that reveal structural details of the molecules. A team from the Max Planck Institute for Medical Research in Germany collaborated with LCLS staff in injecting samples into the path of the X-ray laser pulses.
A goal of the screening test was to invite groups and individuals who hadn't led experiments at LCLS before, Messerschmidt said: "That was the main idea: to get new people in."
Hasan Demirci, an investigator from Brown University who had tried twice before to get a proposal accepted to study delicate crystals of protein-creating ribosomes with LCLS, had been waiting for such an opportunity.
"Before, it was a black box," Demirci said of LCLS. But now, he said, he has a clear view of all of the steps involved in an experiment, from pre-testing to data collection and analysis.
"It was a really great experience," Demirci said. "For small groups, I think this is a great way to get access to LCLS. We can bring new ideas to this endeavor – everybody has a different perspective."
The groups involved in the test runs processed 46 samples, and based on feedback from participants, Messerschmidt said he expects some of them collected enough high-quality data in the limited time available that they expect to publish their results.
Sabine Botha, a doctoral student with the Max Planck Institute team, led the effort to feed samples through sophisticated nozzles into the path of the X-ray pulses. She said the screening trial was logistically challenging but ran "remarkably smoothly."
"There was absolutely no sample that we didn't manage to get into the beam," Botha said. "Quite a few of the scientists got really excited and happy." She added that the tests provided a solid learning experience for newcomers about machine operations and the required quality and purity of samples.
Contact
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.

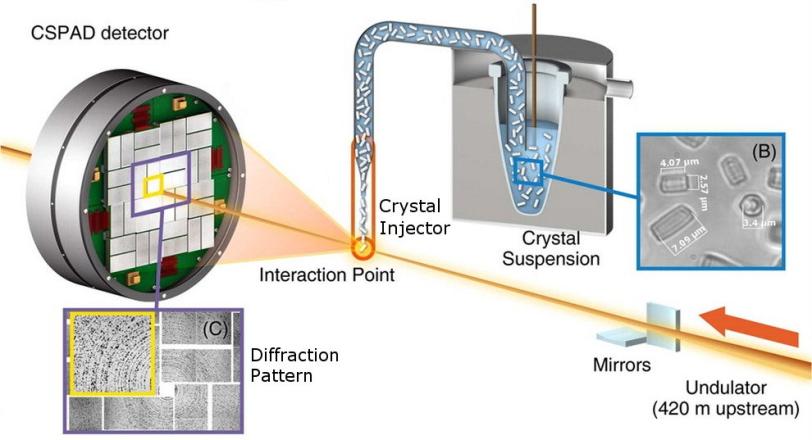
(Adapted from Sierra et al. Acta Crystallogr. D, 2012, D68, 1584-1587)






