Analysis of the rock record rules out atmospheric oxygen before the Great Oxygenation Event
New research questions ‘whiff of oxygen’ in Earth’s early history.
Chemical signatures that have been considered evidence for a “whiff of oxygen” in Earth’s atmosphere before the Great Oxygenation Event 2.3 billion years ago were probably introduced at a much later time, according to research published in Science Advances.
The result rewinds previous research findings and has the potential to rewrite what is known of the planet’s past, including when oxygen-producing bacteria first appeared on Earth.
“Without the whiff of oxygen reported by a series of earlier studies, the scientific community needs to critically reevaluate its understanding of the first half of Earth’s history,” said Dartmouth College earth scientist Sarah Slotznick, the first author of the study.
The study focused on Australia's Mount McRae Shale, samples from which had been interpreted to suggest atmospheric oxygen existed earlier in Earth's history. Slotznick and colleagues found that a series of changes after the shale first formed on the ocean floor were likely responsible for chemical evidence of oxygen.
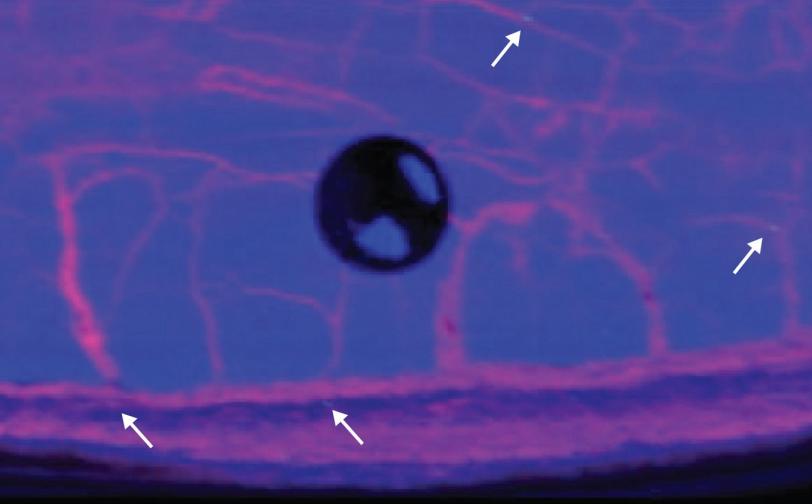
In particular, a new set of tools, including X-ray fluorescence spectroscopy conducted at the Department of Energy's SLAC National Accelerator Laboratory and nanoscale mass spectroscopy performed at the California Institute of Technology, revealed the presence of cracks in the rock where signs of atmospheric oxygen crept in long after the rock itself had formed.
“It’s a really cool problem,” said Samuel Webb, a staff scientist at SLAC’s Stanford Synchrotron Radiation Lightsource and a coauthor on the new study. “It’s taken nine or 10 years to get all the technologies put together to be able to do these measurements, and then we are able to see the composition and the importance of these small cracks and fissures in the rocks,” which revealed how signs of oxygen got into the rock at a later time than its initial formation, when originally there were none.
The initiation of oxygenation
For decades, scientists have debated when measurable levels of oxygen first appeared in Earth’s atmosphere. Over the last century, scientists developed the idea of the Great Oxygenation Event (GOE), a period over 2 billion years ago during which oxygen levels began to increase, paving the way for the rise of complex cells, animals and eventually humans.
More recently, however, research on chemical signals related to oxygen has suggested transient appearances of oxygen, known as “whiffs,” half a billion years earlier.
In 2007, two parallel studies found evidence of such a whiff based on samples of the 2.5-billion-year-old Mount McRae Shale, results that seemed to contradict other indications arguing that no oxygen was present in the atmosphere before the GOE.
A research origin story
Since atmospheric oxygen cannot be measured directly in rock, the 2007 studies were based on evidence of oxygen-related chemical reactions in molybdenum and sulfur within the shale. The findings raised fundamental questions about the early evolution of life on Earth. In particular, they suggested that cyanobacteria, early innovators in photosynthesis, may have appeared much earlier than previously thought.
The new study dates back to 2009, when a Caltech-led team began to conduct additional analysis. The team, some of whom have since moved to other institutions, took over a decade to collect and analyze data, resulting now in the first published study that directly refutes the finding of a whiff of early oxygen.
“Rocks this old tell a complicated story that goes beyond what the world was like when the mud was deposited,” said Caltech geobiologist and study coauthor Woodward Fischer. “These samples also contain minerals that formed long after their deposition when ancient environmental signals were mixed with younger ones, confusing interpretations of the conditions on ancient Earth.”
A matter of approach
The 2007 research papers that found the oxygen whiff analyzed powdered samples from throughout the Mount McRae Shale. This time, rather than conducting a chemical analysis on powder, the researchers inspected specimens of the rock using a series of high-resolution techniques.
First, the research team recorded images on a flatbed optical scanner of rock samples taken from the shale in 2004. Based on those observations, they then collected thin samples for additional analyses. The suite of approaches used on the specimens, including synchrotron-based X-ray fluorescence spectroscopy, gave the team additional insight into the geology and chemistry of the samples as well as the relative timing of processes that were identified.
The new analysis shows that the Mount McRae Shale formed from organic carbon and volcanic dust. The research indicates that molybdenum came from volcanoes and subsequently concentrated during what has been previously identified as the oxygen whiff. As the sediments turned into rock, fracturing created pathways where fluids would bring in chemical signals of oxidation hundreds of millions of years later.
Looking back to point a way forward
In addition to providing an alternate explanation for signs of oxygen that were found in the Mount McRae Shale, the team confirmed that the level of atmospheric oxygen was negligible in the period approximately 150 million years before the GOE.
The findings call into question the early existence of cyanobacteria, instead supporting other hypotheses that oxygen-generating photosynthesis evolved only shortly before the GOE.
“We expect that our research will generate interest both from those studying Earth and those looking beyond at other planets,” said Slotznick. “We hope that it stimulates further conversation and thought about how we analyze chemical signatures in rocks that are billions of years old.”
Editor's Note: This article is based on a press release from Dartmouth College.
The research was supported by the Agouron Institute, NASA, the National Science Foundation, and the Packard Foundation. SSRL is a DOE Office of Science user facility.
Citation: Sarah Slotznick, Science Advances, January 5, 2021 (10.1126/sciadv.abj7190)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
About SLAC
SLAC National Accelerator Laboratory explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by researchers around the globe. As world leaders in ultrafast science and bold explorers of the physics of the universe, we forge new ground in understanding our origins and building a healthier and more sustainable future. Our discovery and innovation help develop new materials and chemical processes and open unprecedented views of the cosmos and life’s most delicate machinery. Building on more than 60 years of visionary research, we help shape the future by advancing areas such as quantum technology, scientific computing and the development of next-generation accelerators.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.





