Room-temperature crystallography aids new study of photosynthetic bacteria
Recently developed methods now in use at SLAC’s X-ray synchrotron helped a team of chemists better understand how certain bacteria turn light into chemical energy.
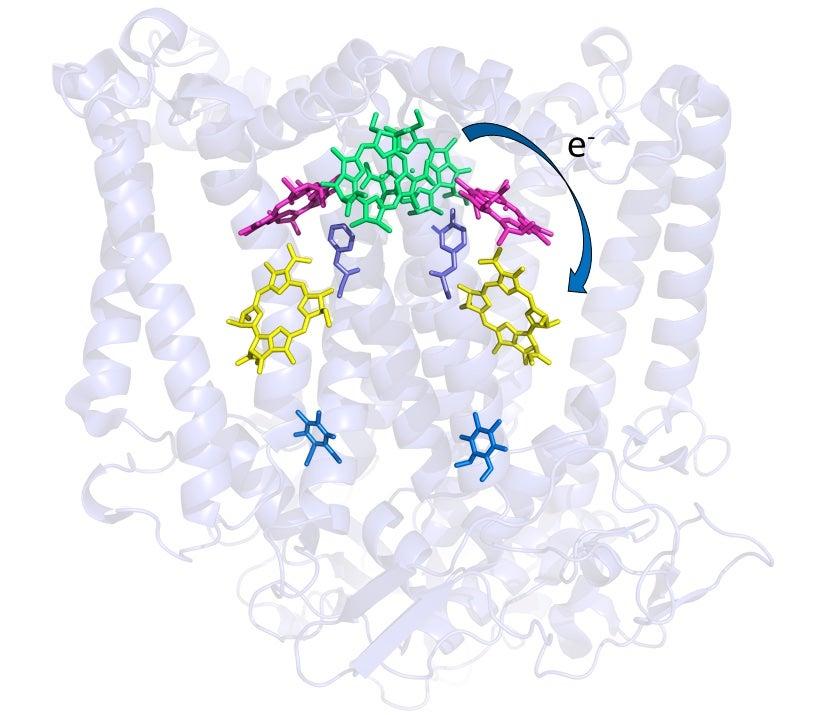
Chemists have come to a deeper understanding of how photosynthetic bacteria convert light into chemical energy and discovered why one step in the process may be more robust than previously realized, according to a new study published this week in Proceedings of the National Academy of Sciences.
The study focused on proteins called reaction centers in a bacterium called Rhodobacter sphaeroides that help transport electrons in its cell membrane during the first steps of photosynthesis. Although these proteins, which reside in the cell membrane, have been studied for decades, many details of how they work remain unclear. To try to fill in some of those details, Stanford University's Jared Weaver, a graduate student in chemist Steven Boxer's laboratory, worked with fellow Stanford chemist Chi-Yun Lin and Washington University researchers Kaitlyn Fairies, Dewey Holten, and Chris Kirmaier, who have been studying R. sphaeroides reaction centers for over a decade. Their approach was to replace part of the protein with amino acids – protein building blocks – that do not naturally appear in that part of the protein structure. The results could help researchers better understand how the electron-transporting protein works under normal operation.
As part of those investigations, Weaver teamed up with Irimpan Mathews, a staff scientist at the Stanford Synchrotron Radiation Lightsource (SSRL) at the Department of Energy's SLAC National Accelerator Laboratory. There, the pair worked to crystallize the modified photosynthetic proteins and study them with X-ray macromolecular crystallography at one of SSRL's beamlines.
Unfortunately, nothing Weaver and Mathews tried seemed to work. They realized that the samples might be getting damaged when they cooled them down to the normal temperature used for X-ray crystallography studies – around 100 Kelvin or -280 degrees Fahrenheit.
With that in mind, Mathews proposed turning to another SSRL scientist, Silvia Russi, who has been developing alternative methods at SSRL that enable studying samples at closer to room temperature, without any freezing at all. Russi's method works by optimizing the humidity in a sample to improve X-ray diffraction power – and hence data-gathering ability – at much warmer temperatures. In this case the team turned to the method simply to get data, but there is another benefit that many researchers find attractive: By operating at near room temperatures, researchers can get data on proteins in a more physiologically relevant context.
By combining room-temperature crystallography with spectroscopy and other techniques, Weaver said, the team was able to get a closer look at how bacterial reaction centers shuttled electrons around during the first steps of photosynthesis. Surprisingly, while seemingly drastic changes to the active site of those proteins affected how well they operated, the drop in efficiency wasn't nearly as big as expected. Taken together, Weaver said, the results yielded new insight into the mechanism of electron transfer early in the process of photosynthesis and showed that reaction centers are "remarkably robust," Weaver said.
The research was supported by the National Science Foundation and the DOE Office of Science. SSRL is a DOE Office of Science user facility. The Structural Molecular Biology Program at SSRL is supported by the DOE Office of Science and by the National Institutes of Health, National Institute of General Medical Sciences.
Citation: Jared Weaver et al., Proceedings of the National Academy of Sciences, 15 December 2021 (10.1073/pnas.2116439118)
Contact
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a vibrant multiprogram laboratory that explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by scientists around the globe. With research spanning particle physics, astrophysics and cosmology, materials, chemistry, bio- and energy sciences and scientific computing, we help solve real-world problems and advance the interests of the nation.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.





