It's so metal: Scientists confirm nickel plays a key role in an ancient chemical reaction
SLAC scientists showed that a carbon-metal compound with a perfectly placed nickel atom plays a key role in converting carbon dioxide into components for food and energy.
By Kimberly Hickok
Carbon dioxide (CO2) is the most abundant greenhouse gas causing climate change but has existed on Earth long before humans started releasing it into the atmosphere at unprecedented levels. As such, some of the planet’s earliest organisms evolved to harness and make use of this gas that is otherwise harmful to humans and the planet.
One of those processes, called the Wood-Ljungdahl pathway, only occurs in the absence of oxygen and is thought to be the most efficient carbon-fixation pathway in nature. But exactly how the pathway proceeds from one step to the next has remained unclear.
Now, scientists at the Stanford Synchrotron Radiation Lightsource (SSRL) at the Department of Energy's SLAC National Accelerator Laboratory, University of Michigan, Northwestern University, and Carnegie Mellon University have discovered the previously unknown inner workings of the Wood-Ljungdahl pathway. Their findings, published in the Journal of the American Chemical Society last month, not only shed light on one of the oldest chemical reactions on Earth, but may also lead to improved carbon capture techniques for climate change mitigation efforts.
“Before this study, we knew that in order for the Wood-Ljungdahl pathway to generate carbon for organisms to use, it starts with carbon dioxide,” said Macon Abernathy, a research associate at SSRL and coauthor of the study. “Then it converts CO2 to carbon monoxide and a methyl group and through some kind of chemistry magic merges them into a form of carbon that can be used by the organism.”
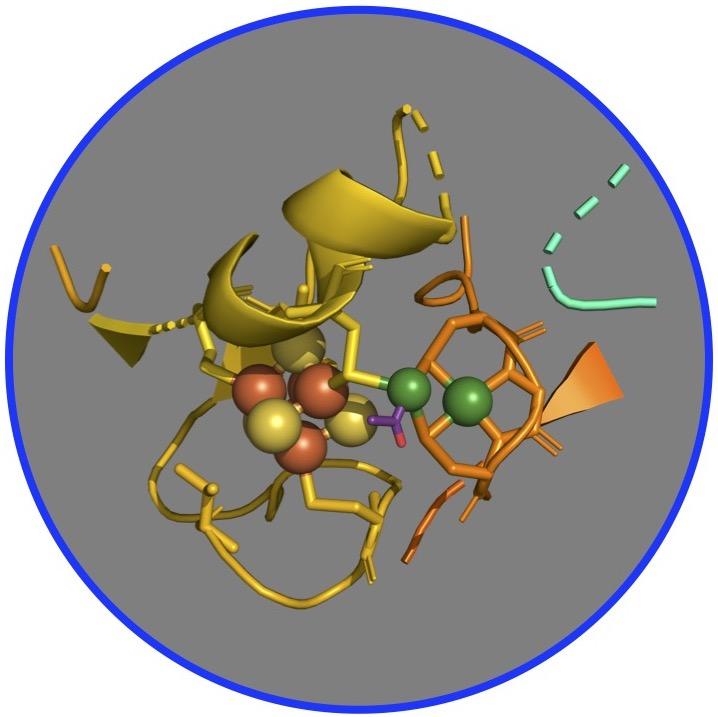
For years, scientists postulated that the pathway operates through a series of nickel-based organometallic intermediates, which form metal-carbon bonds. Specifically, researchers have focused on a complex of two nickel-iron-sulfur proteins, called CO dehydrogenase and acetyl-CoA synthase (CODH/ACS), which are the primary enzymes that catalyze the conversion of carbon dioxide into energy and structural carbon for building cell walls and proteins.
But confirming this hypothesis has proven tricky as the enzyme complex needs to be purified in an atmosphere lacking oxygen, like that of early Earth 4 billion years ago when these proteins and this pathway emerged. Furthermore, the intermediate compounds are often unstable and the reaction can quickly become inactive. In addition, the presence of other atoms of nickel and iron in the CODH interferes with study of ACS, the target of this study.
To circumvent these challenges, the researchers developed a more active, ACS-only version of the protein – no CODH – and used X-rays at SSRL to understand its metals and how they work inside the enzyme. The team applied X-ray spectroscopy, a technique in which scientists study the interference of light that is absorbed by, released from and then bounced back to the metals in a complex – here the ACS – to identify changing chemical bonds as the reactions take place.
In short, the scientists confirmed their long-standing hypothesis.
“What we found is that there is a very intricate bit of organometallic chemistry that's going on where a single nickel site in the enzyme is doing all the fun stuff,” said Ritimukta Sarangi, senior scientist at SSRL and a corresponding author on the study.
The team learned that although the enzyme has a cluster of two nickels bound to four atoms each of iron and sulfur, the reaction always takes place at one specific nickel within the cluster, said Steve Ragsdale, a professor at the University of Michigan and corresponding author on the study. “The carbons, such as carbon monoxide, the methyl group, and the acetyl group, all bind to the nickel closest to the iron and sulfur, and it’s very clear that they don’t bind to any of the other metals.”
The researchers also noticed that the nickel-containing protein undergoes major changes in its structure at each one of the intermediate states, Ragsdale said. “That’s something that was not really part of our original hypothesis. We were just thinking about the basic chemistry being nickel-based. But then we see all these other changes that take place in the protein, which was a little bit surprising.”
Although the researchers had strong ideas about how the reaction works, seeing it in action was nonetheless impressive, said Abernathy.
“It's such precise fine tuning of nature to arrive at this elegant system that does this catalysis,” Sarangi said. “I just love that, and our ability to use X-ray spectroscopy, which is an extremely powerful tool for figuring out what's going on in nature. SSRL’s Structural Molecular Biology Resource has a world-leading biological X-ray spectroscopy program that enables the study of such complex biological processes.”
Besides his appreciation for the natural beauty of the Wood-Ljungdahl pathway itself, Ragsdale said he hopes that research to better understand these natural processes, and perhaps enhance those processes, could lead to methods for mitigating climate change and developing carbon capture to make chemical feedstocks and fuels. “I think that we have to understand the basic biochemistry behind the process first,” he said, “before we'll be able to make progress in enhancing some of these pathways as they exist in nature.”
SSRL is a DOE Office of Science user facility. The research was funded by the DOE Office of Science. SSRL’s Structural Molecular Biology Resource is funded by the National Institutes of Health and DOE Office of Science.
Contact
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
About SLAC
SLAC National Accelerator Laboratory explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by researchers around the globe. As world leaders in ultrafast science and bold explorers of the physics of the universe, we forge new ground in understanding our origins and building a healthier and more sustainable future. Our discovery and innovation help develop new materials and chemical processes and open unprecedented views of the cosmos and life’s most delicate machinery. Building on more than 60 years of visionary research, we help shape the future by advancing areas such as quantum technology, scientific computing and the development of next-generation accelerators.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.