Uncertainty Gives Scientists New Confidence in Search for Novel Materials
Scientists have found a way to estimate uncertainties in computer calculations that are widely used to speed the search for new materials for industry, electronics, energy, drug design and a host of other applications.
Menlo Park, Calif. — Scientists at Stanford University and the Department of Energy’s SLAC National Accelerator Laboratory have found a way to estimate uncertainties in computer calculations that are widely used to speed the search for new materials for industry, electronics, energy, drug design and a host of other applications. The technique, reported in the July 11 issue of Science, should quickly be adopted in studies that produce some 30,000 scientific papers per year.
“Over the past 10 years our ability to calculate the properties of materials and chemicals, such as reactivity and mechanical strength, has increased enormously. It’s totally exploded,” said Jens Nørskov, a professor at SLAC and Stanford and director of the SUNCAT Center for Interface Science and Catalysis, who led the research.
“As more and more researchers use computer simulations to predict which materials have the interesting properties we’re looking for – part of a process called ‘materials by design’ – knowing the probability for error in these calculations is essential,” he said. “It tells us exactly how much confidence we can put in our results.”
Nørskov and his colleagues have been at the forefront of developing this approach, using it to find better and cheaper catalysts to speed ammonia synthesis and generate hydrogen gas for fuel, among other things. But the technique they describe in the paper can be broadly applied to all kinds of scientific studies.
Speeding the Material Design Cycle
The set of calculations involved in this study is known as DFT, for Density Functional Theory. It predicts bond energies between atoms based on the principles of quantum mechanics. DFT calculations allow scientists to predict hundreds of chemical and materials properties, from the electronic structures of compounds to density, hardness, optical properties and reactivity.
Because researchers use approximations to simplify the calculations – otherwise they’d take too much computer time – each of these calculated material properties could be off by a fairly wide margin.
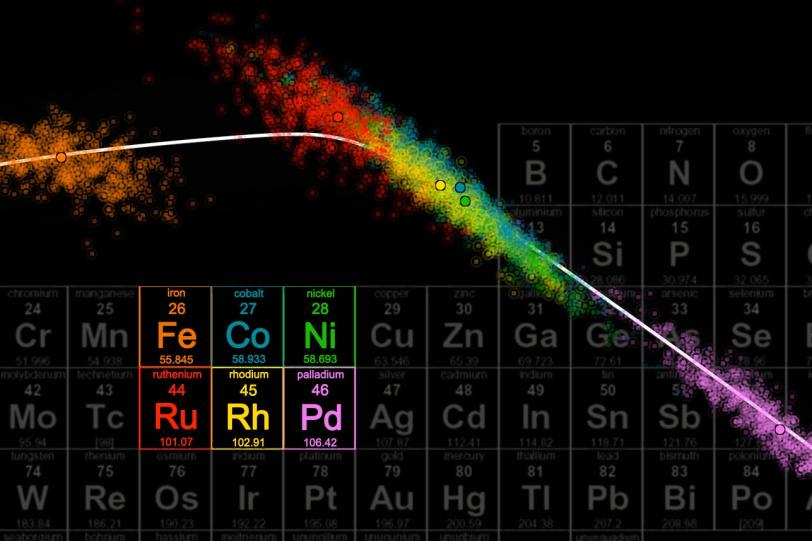
To estimate the size of those errors, the team applied a statistical method: They calculated each property thousands of times, each time tweaking one of the variables to produce slightly different results. That variation in results represents the possible range of error.
“Even with the estimated uncertainties included, when we compared the calculated properties of different materials we were able to see clear trends,” said Andrew J. Medford, a graduate student with SUNCAT and first author of the study. “We could predict, for instance, that ruthenium would be a better catalyst for synthesizing ammonia than cobalt or nickel, and say what the likelihood is of our prediction being right.”
An Essential New Tool for Thousands of Studies
DFT calculations are used in the materials genome initiative to search through millions of solids and compounds, and also widely used in drug design, said Kieron Burke, a professor of chemistry and physics at the University of California-Irvine who was not involved in the study.
“There were roughly 30,000 papers published last year using DFT,” he said. “I believe the technique they’ve developed will become absolutely necessary for these kinds of calculations in all fields in a very short period of time.”
Thomas Bligaard, a senior staff scientist in charge of theoretical method development at SUNCAT, said the team has a lot of work ahead in implementing these ideas, especially in calculations attempting to make predictions of new phenomena or new functional materials.
Other researchers involved in the study were Jess Wellendorff, Aleksandra Vojvodic, Felix Studt, and Frank Abild-Pedersen of SUNCAT and Karsten W. Jacobsen of the Technical University of Denmark. Funding for the research came from the DOE Office of Science.
Citation: A. Medford et al., Science, 11 July 2014 (10.1126/science.1253486)
Press Office Contact: Manuel Gnida, mgnida@slac.stanford.edu, 415-308-7832
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, California, SLAC is operated by Stanford University for the U.S. Department of Energy Office of Science.
The SUNCAT Center for Interface Science and Catalysis is a partnership between SLAC National Accelerator Laboratory and Stanford University. SUNCAT explores the atomic-scale design of catalysts for chemical processes related to energy conversion and storage. For more information, please visit suncat.slac.stanford.edu.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.