Superhard Diamond-Denting Material Created
A superhard mixture of crushed carbon spheres and a hydrocarbon solvent is the world’s first hybrid crystalline/amorphous material.
By Mike Ross
A superhard mixture of crushed carbon spheres and a hydrocarbon solvent is the world’s first hybrid crystalline/amorphous material. Its creation by an international scientific team that included Wendy Mao, a Stanford University professor and SLAC researcher, was announced in this week’s issue of Science magazine.
The new material is one of a class that is hard enough to dent diamond, the hardest known material. The team created it by squeezing a mixture of soccer-ball-shaped carbon-60 molecules (popularly known as “buckyballs”) and a xylene solvent to extremely high pressures – up to 600,000 times atmospheric pressure – in a device called a diamond anvil cell. The cell holds a tiny amount of material that is pressed between the flattened tips of two opposing diamonds. Scientists can shine lasers or X-rays through the transparent diamonds to observe and identify any atomic-scale changes caused by the rising pressure.
These new materials were created at Argonne National Laboratory's Advanced Photon Source by a team mostly associated with the Geophysical Laboratory of the Carnegie Institution of Washington. A former Geophysical Lab scientist herself, Mao has continued to collaborate on projects there since she moved to Stanford and SLAC in 2007.
Unlike earlier pressure-created carbon materials that Mao has created, this one retained its superhard compressed structure after the pressure was released, so it may ultimately find some industrial application, such as a wear-resistant protective coating.
“Although we haven’t yet tested its properties directly, we know it’s superhard because it dented the diamond anvil face,” Mao said. “Our next step is to test this new material’s properties. If they prove desirable, then we’d want to devise an economical way of making it. The diamond anvil cell is a great tool for discovery, but not for high-volume manufacturing.”
Most intriguing scientifically is that this material retained the long-range, regular molecular pattern that is characteristic of crystals even after the intense pressure crushed its major constituents – the buckyballs – into jumbled, amorphous blobs of carbon. The scientists determined that the solvent molecules played a crucial role in preserving the material’s crystallinity.
“Hybridization of crystalline and amorphous structures at an atomic level hasn’t been experimentally observed, although scientists believed such structures could be created,” said the paper’s first author, Carnegie scientist Lin Wang. “The finding in this paper should be the first of its kind.”
Mao is a member of the Stanford Institute for Materials and Energy Sciences, a joint institute of SLAC and Stanford.
For more details, see the group’s paper in Science Magazine or the press release from the Carnegie Institution.
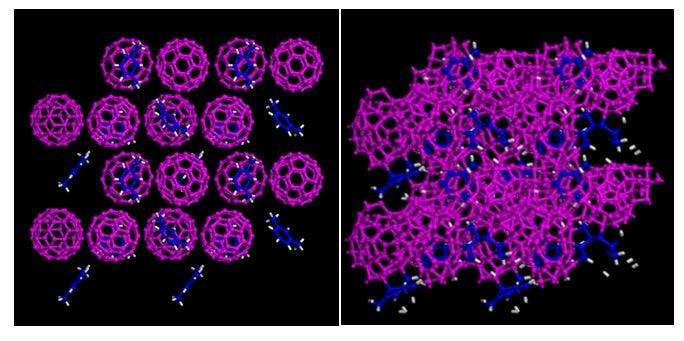
(Image by Lin Wang, Carnegie Institution of Washington)