News Feature
VIA Stanford News
Stanford's Global Climate and Energy Project Awards $9.3 Million for Innovative Energy Research

News Feature
VIA Stanford Energy
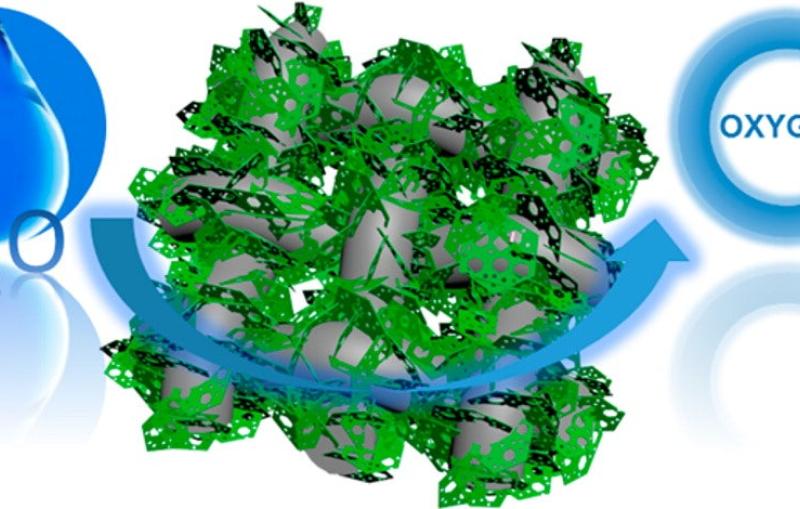
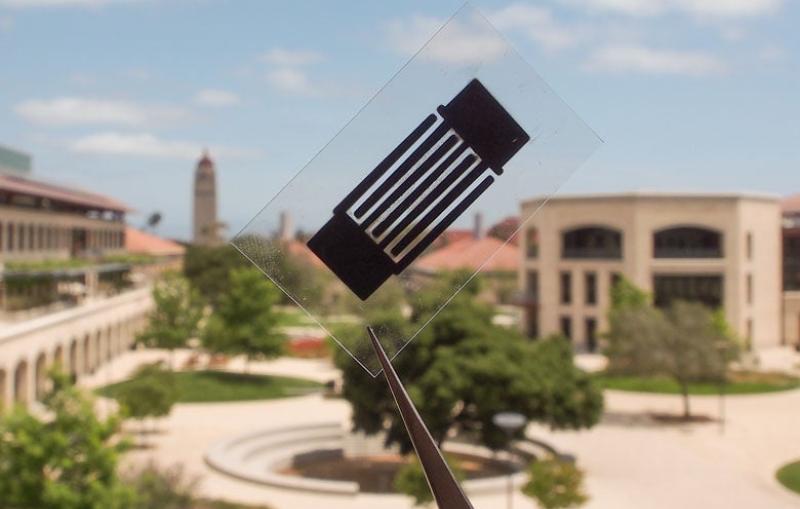
New Fuel-cell Materials Could Pave the Way for Practical Hydrogen-powered Cars