SLAC X-rays Help Discover New Drug Against Melanoma
It was front page news around the world: a drug designed to disrupt malignant melanoma, the deadliest form of skin cancer, was so successful in its latest round of testing in humans that the tests were halted – like an early-round knockout in boxing – so patients in the trial who were receiving other treatments could be moved to the new medicine.
By Mike Ross
It was front page news around the world: a drug designed to disrupt malignant melanoma, the deadliest form of skin cancer, was so successful in its latest round of testing in humans that the tests were halted – like an early-round knockout in boxing – so patients in the trial who were receiving other treatments could be moved to the new medicine.
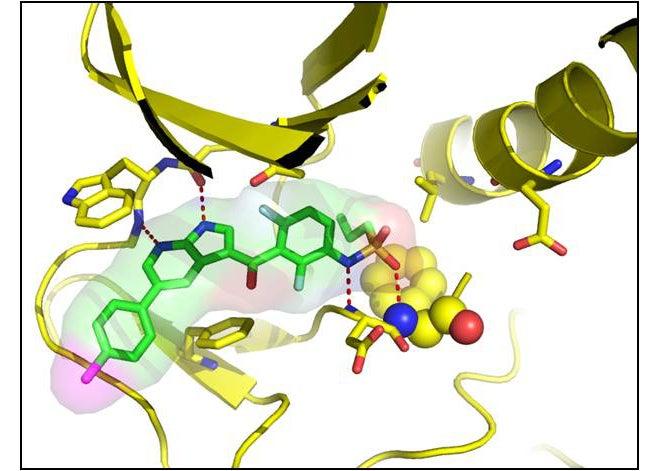
A crucial part of the research for developing this new drug, called vemurafenib, took place at three DOE national laboratories: SLAC National Accelerator Laboratory, Argonne National Laboratory and Lawrence Berkeley National Laboratory. A Berkeley-based drug-discovery company, Plexxikon, used the labs’ powerful X-ray facilities to determine the precise structure of a mutated protein involved in this cancer – and potential drug candidates that could stop its spread.
Plexxikon’s success, reported last month, is an impressive victory for an emerging approach to combating illness: creating drugs custom-designed to throw molecular monkey wrenches into the disease process.
First researchers identify a target protein that plays a key role in the disease. In this case, it was an enzyme involved in cell growth that sometimes mutates and makes cells multiply out of control, the hallmark of cancer. If the scientists could find a small molecule that fit perfectly into a specific place in the mutated enzyme, they could block the enzyme’s action and slow or stop the cancer.
The researchers screen hundreds of small molecules – potential drugs – and identify the most promising ones. Then they bind each molecule to the target protein, crystallize the bound pair and study it with powerful beams of X-rays, which scatter off the atoms in the crystal and reveal its 3-D structure. This technique, known as macromolecular X-ray crystallography, has become an important tool for probing large, complex biological molecules and discovering new drugs. Molecules that look like good blockers are chemically tweaked to optimize their performance, and their structures determined again to see if they bind to the target protein more effectively. It may take several rounds of such chemical tinkering and X-ray structure work to find the optimal molecule for stopping the disease with no significant side effects. Drug candidates then undergo a rigorous series of highly-regulated trials to determine their effectiveness, safety, side effects and proper dosages.
Plexxikon also used the X-ray crystallography facilities at SLAC’s Stanford Synchrotron Radiation Lightsource, or SSRL, to design two other drugs that are now being tested in humans. One is aimed at type II diabetes and other metabolic disorders. The other attacks cells found in many metastatic breast, colorectal, lung, and prostate cancers, and may also be effective against autoimmune diseases such as rheumatoid arthritis, multiple sclerosis and lupus.
“Our unique success story in producing a life-changing drug in a matter of a few years is a testament to the power of structural information,” said Chao Zhang, the company’s head of structural chemistry. He said structures produced by X-ray crystallography “provide precise information on how a drug interacts with its protein target. This information points us in the most productive directions, enabling a small chemistry team to generate new drug candidates quickly.”
Macromolecular crystallography is a rapidly growing activity. Six of SSRL’s 30 beamlines host a large number of such projects each year; the specialized beam lines are funded primarily by the Department of Energy’s Office of Biological and Environmental Research and the National Institutes of Health. Worldwide, scientists are using more than 130 X-ray synchrotron beamlines to study biological molecules. X-ray crystallography was used to determine some 87 percent of the nearly 74,000 structures submitted to date to the Protein Data Bank, the worldwide repository for 3-D structure information on large biological molecules, and the vast majority of those used synchrotron X-rays.
Since scientists first demonstrated the feasibility of using synchrotron radiation for macromolecular crystallography at SSRL in 1976, greater X-ray intensity, better beam quality and improved detectors, computers and sample-handling automation have dramatically increased the speed and accuracy with which scientists can obtain their results.
“Ten years ago we could typically examine only 20 crystals per 8-hour shift on a beamline, and collect data from one or two of them,” said Ana Gonzalez, SSRL senior staff scientist. Now, she said, users can manipulate their samples and measure the data remotely, over the Internet, “and during a single shift we can look at some 100 crystals and also collect datasets from 20 to 30 of them.”
The future offers potentially transformative new technologies thanks to SLAC’s Linac Coherent Light Source, or LCLS. In experiments there, an international team of scientists showed that they can get the rough 3-D structures of proteins from tiny protein nanocrystals, which may be much easier to create than the larger crystals needed for traditional synchrotron-based X-ray diffraction. The nanocrystals are suspended in water and squirted through the powerful LCLS X-ray laser beam, which pulses 120 times a second. In the instant before the intense X-rays destroy a nanocrystal, detectors record a flash of X-ray diffraction information. Finally, scientists use sophisticated computer programs to merge the data from hundreds of thousands of nanocrystals to reveal the protein’s structure.
By enabling scientists to determine the structures of many proteins that don’t fully crystallize, “the ultrafast LCLS X-ray beam has the potential to guide the design of next-generation drugs,” said Plexxikon’s Zhang.