Tick, Tock on the ‘Attoclock’: Tracking X-Ray Laser Pulses at Record Speeds
When it comes to making molecular movies, producing the world’s fastest X-ray pulses is only half the battle. A new technique reveals details about the timing and energy of pulses that are less than a millionth of a billionth of a second long.
By Amanda Solliday and Angela Anderson
To catch chemistry in action, scientists at the Department of Energy’s SLAC National Accelerator Laboratory use the shortest possible flashes of X-ray light to create “molecular movies” that capture the motions of atoms in chemical reactions and reveal new details about the most fundamental processes in nature.
Future experiments at the Linac Coherent Light Source (LCLS), SLAC’s X-ray free-electron laser, will use pulses that last just attoseconds (billionths of a billionth of a second). Such experiments will be even more powerful because they'll be able to detect the motions of electrons within molecules during chemical reactions. However, to design such ultrafast experiments, researchers need meticulous measurements of the X-ray pulses so they can use that information to interpret the data they collect on the samples they study.
Now an international team, including SLAC scientists, has created an X-ray “attoclock” that lets them analyze X-ray pulses on the attosecond timescale of electron motions.
“Using this method, we can resolve details of the pulses in the attosecond domain for the first time,” says Ryan Coffee, a senior scientist at LCLS and the Stanford PULSE Institute and a principal investigator on the team. “This paves the way for X-ray free-electron laser science at a timescale that is key to understanding physical chemistry.”
The team’s research was published in Nature Photonics on March 5.
Timekeeping in Attoseconds
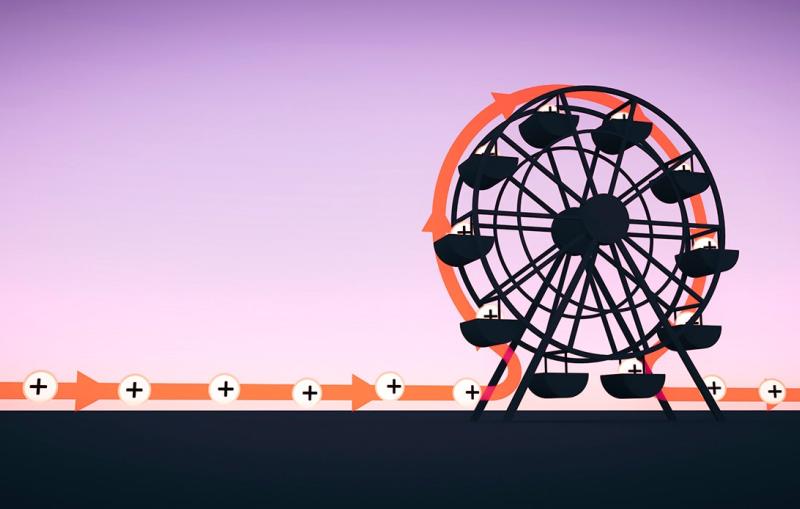
The term “attoclock” was coined by Swiss physicist Ursula Keller, who first demonstrated a technique to study attosecond processes with circularly polarized light 10 years ago. However, the LCLS version is the first one designed to measure individual X-ray pulses, one by one.
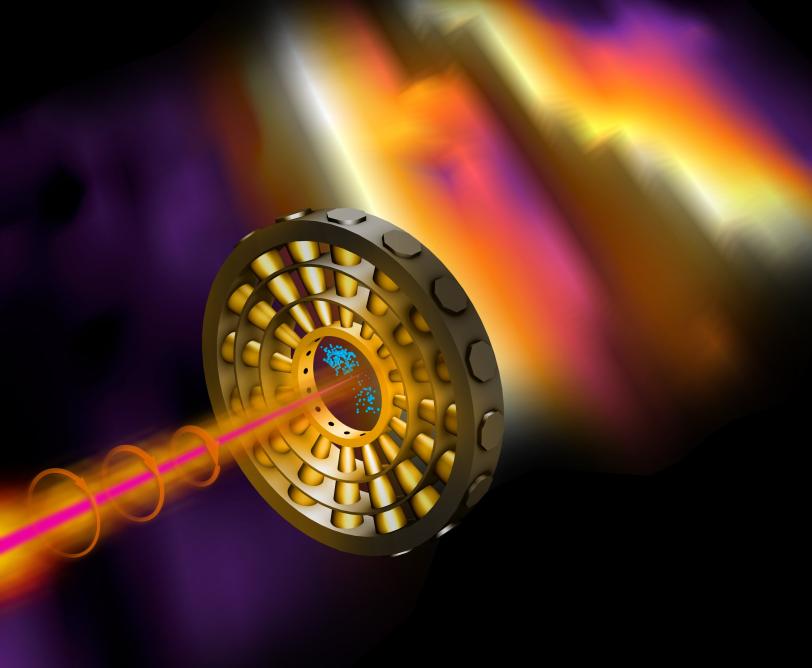
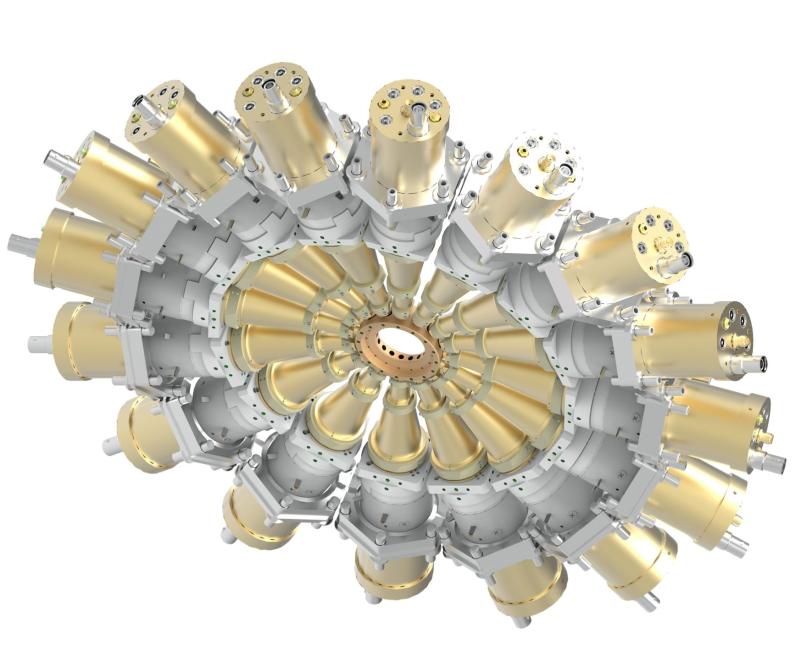
It consists of a ring of detectors arranged like numbers on the face of a clock. When an X-ray pulse hits a target at the center of the clock, it knocks electrons out of the target's atoms. Those electrons are hit by circularly polarized laser light that whirls the electrons around the ring before they land on one of the detectors. The position of that detector – the number on the clock face – tells scientists how much energy the X-ray pulse contained and when exactly it hit the target.
“It’s like reading a watch,” Coffee says. “An electron may strike a detector positioned at one o’clock or three o’clock or anywhere around the clock face. We can tell from where it hits exactly when it was generated by the X-ray pulse.”
In an experiment designed to test the technique, the researchers hit neon gas with an attosecond X-ray pulse and then read which of the 16 detectors arrayed around the attoclock the freed electrons hit.
“In coming up with this technique, we combined ideas from different fields,” says principal investigator Wolfram Helml, then a Marie Curie research fellow at SLAC and the Technical University of Munich and now at the Ludwig-Maximilian University of Munich. “For our purposes, it just made sense to combine the circularly polarized light used in the original attoclock with a ring-shaped detector that has been used in other kinds of experiments.”
Finding the True Colors
The technique will be especially important for pump-probe experiments, in which a molecule is first excited with a “pump” pulse and then analyzed by a second “probe” pulse to see how it reacted.
As short as they are, these pulses can contain many different colors or wavelengths. “The color can also vary widely from pulse to pulse, and our technique can sift through the pulses, finding those that are interesting for the experiment,” Coffee says, noting the importance that such sifting will have for the data deluge expected when an upgrade to the X-ray laser, LCLS-II, comes online a couple years from now. With pulses that arrive up to a million times per second, LCLS-II will produce as much data in a few minutes as LCLS currently collects in a month.
“For instance, only a certain color may excite a molecule when it is ‘pumped,’” Helml said. “With the attoclock we can see what part of the pulse is actually exciting the molecule because we know exactly when particular colors of light arrive. This lets us pinpoint more precisely when changes occur in the molecule as a result of the interaction with light.”
What’s more, scientists can potentially excite individual elements in separate parts of the molecule at the same time using different colors of X-rays.
“With this technique we could look within a single molecule at the interplay between atoms. For example, what’s going on with an oxygen atom and how might that affect the chemical environment surrounding a nearby nitrogen atom?” Helml says. “With that level of detail, we can discern completely new chemical behavior.”
Progress in Motion
The attoclock team is now working on a proposal to build more refined detectors.
“With the next detector, we are aiming to precisely identify a broader spectrum of energies,” Coffee says. “This will be an important feature for our upgraded X-ray laser, LCLS-II, which will produce pulses with an even wider energy range and more multi-color flexibility than our current machine.”
This is one of several ideas being tested at SLAC to give scientists detailed information about attosecond pulses. Two other teams are building similar systems with different types of detectors, including one at LCLS and PULSE that recently published a study in Optics Express.
The international team on the Nature Photonics study also included scientists from Deutsches Elektronen-Synchrotron (DESY) and the European X-ray Free-electron Laser (Eu-XFEL), both in Germany, who also provided the unique ring-shaped detector; University of Kassel in Germany; University of Gothenburg in Sweden; University of Bern in Switzerland; University of Colorado at Boulder; University of the Basque Country in Spain; and Lomonosov Moscow State University in Russia.
LCLS is a DOE Office of Science user facility.
Citation: N. Hartmann et al., Nature Photonics, 5 March 2018 (10.1038/s41566-018-0107-6)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
The Stanford PULSE Institute is a joint institute of SLAC National Accelerator Laboratory and Stanford University. PULSE seeks to advance the frontiers of ultrafast science, with particular emphasis on research using SLAC's Linac Coherent Light Source (LCLS). For more information, please visit www.stanford.edu/group/pulse_institute.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.