Scientists Take First Dip into Water’s Mysterious ‘No Man’s Land’
Scientists at the Department of Energy's SLAC National Accelerator Laboratory have made the first structural observations of liquid water at temperatures down to minus 51 degrees Fahrenheit, within an elusive “no man’s land” where water’s strange properties are super-amplified.
Menlo Park, Calif. — Scientists at the Department of Energy’s SLAC National Accelerator Laboratory have made the first structural observations of liquid water at temperatures down to minus 51 degrees Fahrenheit, within an elusive “no man’s land” where water’s strange properties are super-amplified.
The research, made possible by SLAC’s Linac Coherent Light Source (LCLS) X-ray laser and reported June 18 in Nature, opens a new window for exploring liquid water in these exotic conditions, and promises to improve our understanding of its unique properties at the more natural temperatures and states that are relevant to global ocean currents, climate and biology.
Scientists have known for some time that water can remain liquid at extremely cold temperatures, but they’ve never before been able to examine its molecular structure in this zone.
“Water is not only essential for life as we know it, but it also has very strange properties compared to most other liquids,” said Anders Nilsson, deputy director of the SUNCAT Center for Interface Science and Catalysis, a joint SLAC/Stanford institute, and leader of the research. “Now, thanks to LCLS, we have finally been able to enter this cold zone that should provide new information about the unique nature of water.”
Not Your Typical Liquid
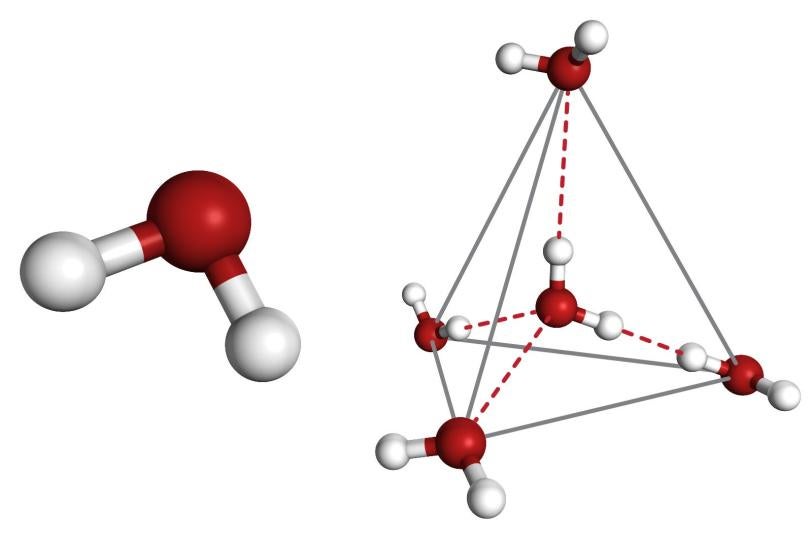
Despite its simple molecular structure, water has many weird traits: Its solid form is less dense than its liquid form, which is why ice floats; it can absorb a large amount of heat, which is carried long distances by ocean currents and has a profound impact on climate; and its peculiar density profile prevents oceans and lakes from freezing solid all the way to the bottom, allowing fish to survive the winter.
These traits are amplified when purified water is supercooled. When water is very pure, with nothing to seed the formation of ice crystals, it can remain liquid at much lower temperatures than normal. The temperature range of water from about minus 42 to minus 172 degrees (see diagram) has been dubbed no man’s land. For decades scientists have sought to better explore what happens to water molecules at temperatures below minus 42 degrees, but they had to rely largely on theory and modeling.
[[{"fid":"7476","view_mode":"crop_wysiwyg_original","type":"media","attributes":{"height":1000,"width":968,"style":"width: 387px; height: 400px; float: left; margin-left: 10px; margin-right: 10px;","alt":"Diagram illustrating water's phases","title":""No Man's Land" Diagram","class":"media-element file-crop-wysiwyg-original"}}]]
This diagram illustrates the rough boundaries of “no man’s land,” a temperature region where supercooled water is difficult to study because of rapid ice formation. Using SLAC’s Linac Coherent Light Source, scientists dipped down to minus 51 degrees Fahrenheit and made the first structural measurements of liquid water in this mysterious region, where water’s unusual properties are amplified. (Greg Stewart/SLAC, Ultrafast Chemical Physics Group/University of Glasgow, Scotland)
Femtosecond Shutter Speeds
Now the LCLS, with X-ray laser pulses just quadrillionths of a second long, allows researchers to capture rapid-fire snapshots showing the detailed molecular structure of water in this mysterious zone the instant before it freezes. The research showed that water's molecular structure transforms continuously as it enters this realm, and with further cooling the structural changes accelerate more dramatically than theoretical models had predicted.
For this experiment, researchers produced a steady flow of tiny water droplets in a vacuum chamber. As the drops traveled toward the laser beam, some of their liquid rapidly evaporated, supercooling the remaining liquid. (The same process cools us when we sweat.) By adjusting the distance the droplets traveled, the researchers were able to fine-tune the temperatures they reached on arrival at the X-ray laser beam.
Colder Still
Nilsson’s team hopes to dive to even colder temperatures where water morphs into a glassy, non-crystalline solid. They also want to determine whether supercooled water reaches a critical point where its unusual properties peak, and to pinpoint the temperature at which this occurs.
“Our dream is to follow these dynamics as far as we can,” Nilsson said. “Eventually our understanding of what’s happening here in no man’s land will help us fundamentally understand water in all conditions.”
Scientists at SLAC’s Linac Coherent Light Source, Stanford Synchrotron Radiation Lightsource and Stanford PULSE Institute; Stockholm University; Germany’s DESY laboratory; the Helmholtz Center for Materials and Energy in Germany; and Stony Brook University in New York also contributed to the research. The work was partially funded by the U.S. Department of Energy Office of Science, the SLAC Laboratory Directed Research and Development Program and the Swedish Research Council.
Citation: J. A. Sellberg et al., Nature, 19 June 2014 (10.1038/nature13266)
Press Office Contact: Andy Freeberg, afreeberg@slac.stanford.edu, (650) 926-4359
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, California, SLAC is operated by Stanford University for the U.S. Department of Energy Office of Science.
SLAC’s LCLS is the world’s most powerful X-ray free-electron laser. A DOE Office of Science national user facility, its highly focused beam shines a billion times brighter than previous X-ray sources to shed light on fundamental processes of chemistry, materials and energy science, technology and life itself. For more information, visit lcls.slac.stanford.edu.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.