First detailed look at how charge transfer distorts a molecule’s structure
Charge transfer is highly important in most areas of chemistry, including photosynthesis and other processes in living things. A SLAC X-ray laser study reveals how it works in a molecule whose lopsided response to light has puzzled scientists for nearly a decade.
By Glennda Chui
When light hits certain molecules, it dislodges electrons that then move from one location to another, creating areas of positive and negative charge. This “charge transfer” is highly important in many areas of chemistry, in biological processes like photosynthesis and in technologies like semiconductor devices and solar cells.
Even though theories have been developed to explain and predict how charge transfer works, they have been validated only indirectly because of the difficulty of observing how a molecule’s structure responds to charge movements with the required atomic resolution and on the required ultrafast time scales.
In a new study, a research team led by scientists from Brown University, the Department of Energy’s SLAC National Accelerator Laboratory and the University of Edinburgh used SLAC’s X-ray free-electron laser to make the first direct observations of molecular structures associated with charge transfer in gas molecules hit with light.
Molecules of this gas, called N,N′-dimethylpiperazine or DMP, are normally symmetric, with a nitrogen atom at each end. Light can knock an electron out of a nitrogen atom, leaving a positively charged ion known as a “charge center.”
Intriguingly, this process is uneven; light absorption creates a charge center in just one of the two nitrogen atoms, and this charge imbalance deforms the molecule’s atomic scaffolding, so atoms compensate by shifting position with respect to each other. But within three trillionths of a second, the charge redistributes itself between the two nitrogen atoms until it evens out and the molecules become symmetric again, the researchers report in a paper published in the Proceedings of the National Academy of Sciences today.
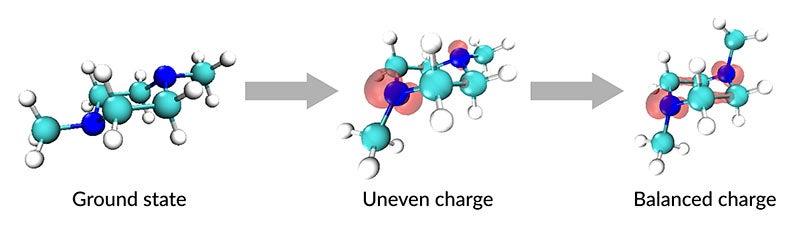
Their study is the first to directly observe how a molecule’s structure changes as charge is redistributed, with some chemical bonds getting longer and some shorter, before finally relaxing back into its original state.
“We see the molecules breaking symmetry and reforming symmetry,” said Peter Weber, a chemistry professor at Brown University whose research group started studying DMP almost a decade ago. He led the study with Adam Kirrander of the University of Edinburgh and SLAC senior staff scientist Michael Minitti.
A lopsided response
Scientists in Weber’s group, including Xinxin Cheng – a PhD student who is now a SLAC associate staff scientist – discovered the molecule’s lopsided response to light eight years ago. It turned out that the molecule’s nitrogen atoms are just the right distance apart to make it an ideal model for studying charge transfer, a discovery that triggered a lot of discussion among theorists working to understand these processes as well as efforts to observe them in more detail.
In this latest study, Haiwang Yong, a PhD student in Weber’s lab, worked with SLAC scientists to provide a much more direct observation of DMP’s response to light. They hit DMP gas with pulses of light followed by extremely short, ultrabright X-ray laser pulses from the lab’s Linac Coherent Light Source (LCLS). The LCLS X-rays scattered off the molecules in a way that revealed the positions of individual atoms, the lengths of the bonds between them and how they changed over just a few trillionths of a second.
“It is fascinating to see how the X-rays can resolve the changes in molecular structure that arise from charge transfer,” Kirrander said.
Weber said the results demonstrate the value of the technique for extracting more detailed information than in previous experiments. The research team used that information to test theoretical models of how molecules respond, revealing flaws in the conventional approach known as density functional theory. Weber noted that the data seems to support detailed theoretical calculations of how these charge transfers take place by Hannes Jónsson of the University of Iceland, who was not involved in this study.
Minitti, who has been working on DMP with the Brown lab from the start and participated in this study, said it has been difficult to get a theoretical understanding of how these asymmetric systems work because the experimental data on them has been so sparse and indirect.
“This work is a significant step forward,” he said, “giving us critical information about how the molecule responds during the charge transfer process. Research like this takes a village – we need experiments to inform the theory, and vice versa, to help us visualize this thing.”
Going forward, a big increase in the pulse repetition rate of the LCLS X-ray source is underway, with a leap from 120 pulses per second to 1 million pulses per second. This will allow researchers to study much more complex systems, informing the development of new approaches to solar energy generation and energy storage technologies, among many other applications.
LCLS is a DOE Office of Science user facility, and the Office of Science funded this work.
Citation: Haiwang Yong et al., Proceedings of the National Academy of Sciences, 3 May 2021 (10.1073/pnas.2021714118)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a vibrant multiprogram laboratory that explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by scientists around the globe. With research spanning particle physics, astrophysics and cosmology, materials, chemistry, bio- and energy sciences and scientific computing, we help solve real-world problems and advance the interests of the nation.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.