IBM/SLAC Team Controls Switching Behavior of Promising Electronic Material
Working with a metal oxide that shows promise for future generations of electronic devices, IBM and SLAC scientists have shown they can precisely control the temperature at which it flips from being an electrical conductor to an insulator – and thus functions as an electronic switch.
By Mike Ross
Working with a metal oxide that shows promise for future generations of electronic devices, IBM and SLAC scientists have shown they can precisely control the temperature at which it flips from being an electrical conductor to an insulator – and thus functions as an electronic switch.
The result is "an important step forwards in the development of future energy-efficient electronic devices," wrote Takashi Mizokawa of the University of Tokyo, who was not part of the research, in a commentary accompanying the team's recent report in Nature Physics.
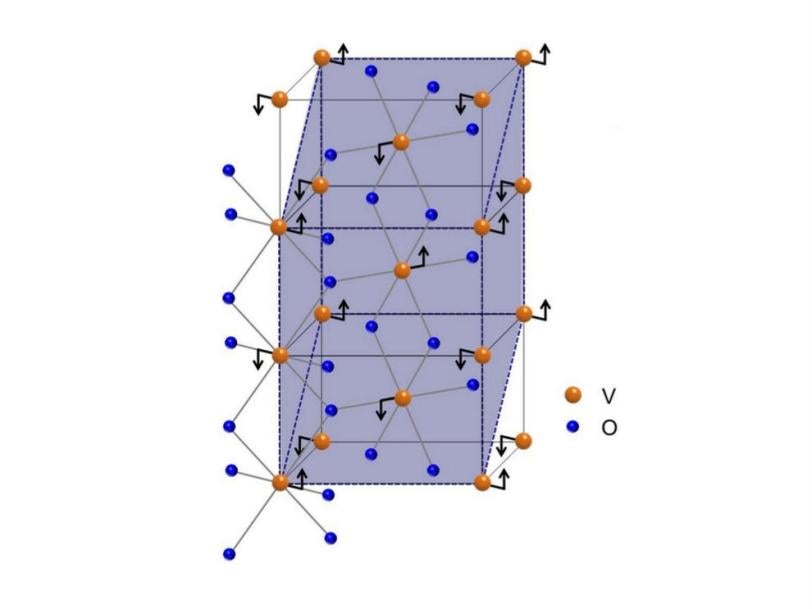
Led by Stuart Parkin of IBM, the team worked with vanadium dioxide, a material whose metal-to-insulator transition behavior has been studied for decades because of its potential for use in transistors and other nanoelectronic switches. It has two big advantages: It switches much faster than traditional transistor materials and it does so at near-room temperature.
Until now, however, researchers did not know how to control the temperature at which this occurs, which is essential to its widespread use.
The IBM/SLAC team was able to control the temperature by growing thin films of vanadium dioxide on a substrate in a way that compressed or stretched the film's crystalline structure, or lattice. As a result, the distance between vanadium atoms in the lattice ranged from 0.5 percent shorter to 1.5 percent longer than normal. This, in turn, slightly changed the arrangement of electrons around the nuclei of vanadium atoms in the lattice.
The researchers found that over the 2 percent change in lattice spacing, the material's metal-insulator switching temperature decreased by more than 100 degrees Fahrenheit, from 152 to 44 degrees Fahrenheit.
X-ray experiments at Lawrence Berkeley National Laboratory showed a direct correlation between the amount of lattice strain and changes in both the arrangement of electrons and the metal-insulator switching temperature.
"The 100-degree temperature difference is a huge effect," said Hermann Durr, who led the team of SLAC scientists performing the X-ray experiments. "X-rays are the ideal tools to discern the interplay between a crystal lattice and electrons. These results will help us design specific materials structures having desired transition temperatures."
Parkin says vanadium dioxide switches could ultimately be used to reduce the leakage of electrical current in conventional transistors – a problem that's been increasing as device dimensions have shrunk – or in entirely new generations of computer circuit elements designed to take advantage of the material's fast metal-insulator transition.
As follow-on research, the IBM/SLAC teams will next use SLAC's Linac Coherent Light Source to learn why the material switches so quickly.
Citations: