Ribosome Research Takes Shape at SLAC
In a new state-of-the-art lab at SLAC National Accelerator Laboratory, components of ribosomes – tiny biological machines that make new proteins and play a vital role in gene expression and antibiotic treatments – form crystals in a liquid solution.
By Glenn Roberts Jr.
In a new state-of-the-art lab at SLAC National Accelerator Laboratory, components of ribosomes – tiny biological machines that make new proteins and play a vital role in gene expression and antibiotic treatments – form crystals in a liquid solution.
Signs at the lab's entryway warn of the potential for contamination – these delicate samples can be damaged by human touch, a sneeze or a dust particle.
"Ribosomes are more fragile than typical protein crystals," said Hasan Demirci, a visiting investigator from Brown University who set up the ultraclean laboratory at SLAC this year to grow the ribosome crystals. "They are like marshmallows." Since they have far fewer sides, or facets, than most crystallized proteins, their structure, or lattice, is easily punctured and wrecked. "You have to be so careful when you harvest them or they will lose their quality," he said.
While the function of ribosomes is well understood, the details of how they work are not, and Demirci hopes his work at SLAC will supply new insights into the timing and chronology of ribosomes at work.
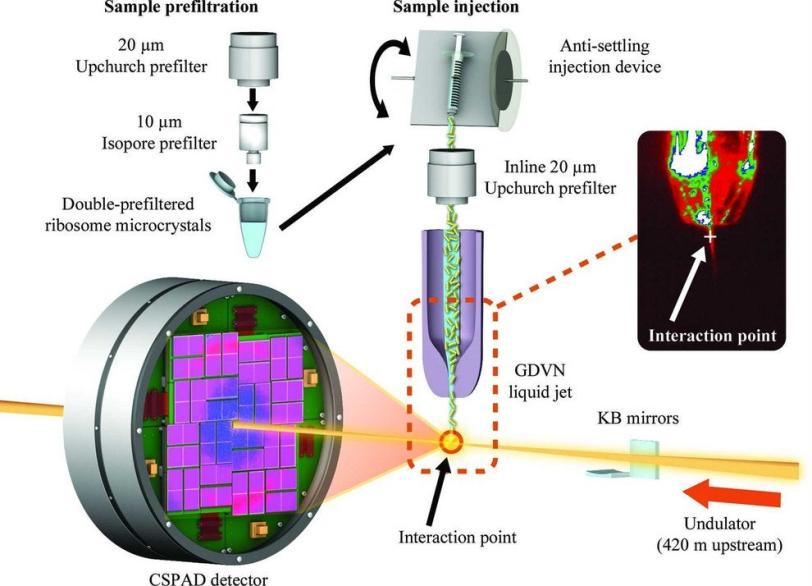
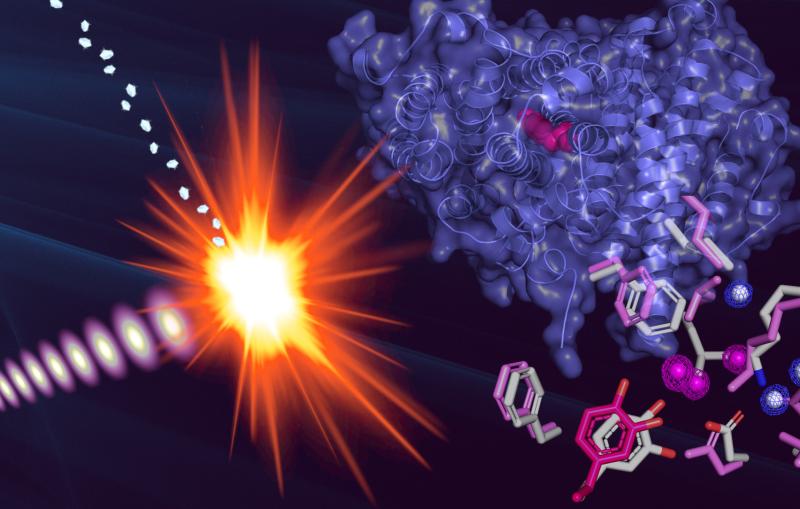
His work got an important boost in March, when he was selected to participate in a test program to study his crystals at SLAC's Linac Coherent Light Source X-ray laser for the first time.
The ultrashort, ultrabright X-ray pulses at LCLS can be used to explore the structure and other properties of crystallized samples in the instant before they are destroyed. LCLS can also be used to study very small crystals, at room temperature and in a liquid solution.
Much of the previous research on ribosomes, carried out at synchrotron X-ray facilities, has focused on larger, frozen samples, though this frozen state can mask their natural features.
Demirci's results, published Aug. 19 in Acta Crystallographica Section F: Structural Biology and Crystallization Communications, demonstrate ultrafast crystallography and the potential for higher-resolution imaging of ribosome crystals from a strain of bacteria, and Demirci hopes the results will lead to more X-ray laser research. "I think with smaller crystals we will get even better resolution," he said. His team is also working to adapt a liquid microjet to deliver the delicate ribosome crystals for study in future experiments.
The results represent the first published work from the protein crystal screening test program at the CXI instrument at LCLS.
Demirci said SLAC's unique combination of research facilities, including LCLS and the Stanford Synchrotron Radiation Lightsource, was a key reason for moving his research to SLAC.
He is particularly interested in studying how one component of ribosomes – the part responsible for deciphering genetic information, which he refers to as the "brain" – reacts to antibiotics.
Many antibiotics are designed to bind with ribosomes in bacterial cells and block protein synthesis, and there are many unknowns about exactly how, when and where this happens. "We want to generate movies of all of those steps," Demirci said.
A better understanding of the binding process could lead to improved antibiotics that are more selective in the way they inhibit ribosome performance and kill bacteria without harming the human host or making the bacteria drug resistant, Demirci said.
"The biology we're after is absolutely the most fundamental," he said.
The work involved a collaboration of scientists from Brown University, SLAC, the Max-Planck Institute for Medical Research, Arizona State University and the Center for Free-Electron Laser Science.