Synchrotrons Explore Water's Molecular Mysteries
In experiments at SLAC National Accelerator Laboratory and Lawrence Berkeley National Laboratory, scientists observed a surprisingly dense form of water that remained liquid well beyond its typical freezing point.
By Glenn Roberts Jr.
In experiments at SLAC National Accelerator Laboratory and Lawrence Berkeley National Laboratory, scientists observed a surprisingly dense form of water that remained liquid well beyond its typical freezing point.
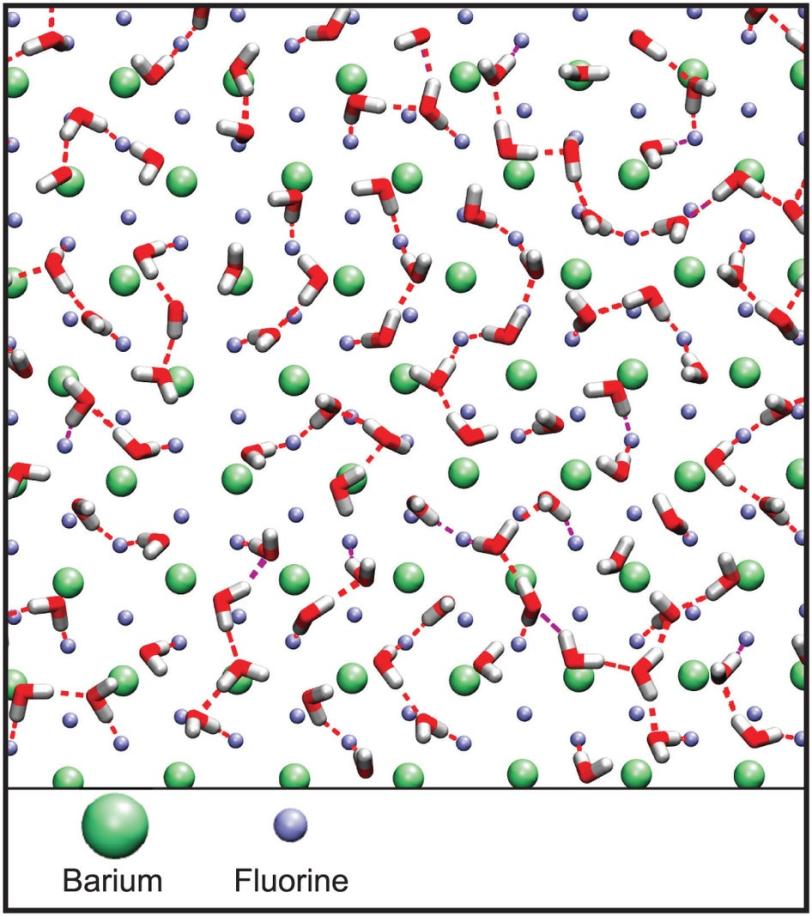
Researchers applied a superthin coating of water – no deeper than a few molecules – to the surface of a barium fluoride crystal.
This surface was expected to stimulate ice formation, but even when chilled to a temperature of about 6.5 degrees Fahrenheit – well below water’s normal freezing point – the water remained liquid.
Further, the molecular structure of the water on the crystal surface unexpectedly transformed to a high-density form in a broad temperature range, mimicking the density water achieves when pressure is applied.
The research, published Jan. 15 in Nature Scientific Reports, spanned more than three years and included experiments at SLAC's Stanford Synchrotron Radiation Lightsource and Berkeley Lab's Advanced Light Source synchrotrons, as well as computer simulations by collaborators in Sweden.
The work represents a milestone in understanding some of the many exotic properties water exhibits under a range of conditions, said Anders Nilsson, one of the lead authors. He is deputy director of the SUNCAT Center for Interface Science and Catalysis, a Stanford/SLAC institute, and a professor of photon science at SLAC.
Understanding the effect that certain materials have on water at the molecular scale may help scientists design materials that "can steer the water structure and properties," he said.
"This can lead to the design of new membranes for water purification," Nilsson said. "Access to clean water will be the next crisis in the world after energy, and maybe even become more challenging."
The paper is the latest in a series of reports on research at SLAC that is exploring the many unique and unusual properties of water: Its solid form is less dense than its liquid form, it can remain liquid well below its typical freezing point and its strong surface tension allows some insects to actually walk on water, for example.
Further studies using different materials are needed to determine whether the high-density form of water observed on the crystal surface is specifically caused by its interaction with that material, or whether it is a general phenomenon, Nilsson said.
The research does refute "widely believed concepts" that the pattern of the crystal's surface, which is similar to the latticed molecular structure of ice, can greatly impact ice formation by serving as a sort of artificial template, he said.
The research team included scientists from SUNCAT, the Department of Geological and Environmental Sciences at Stanford University, Lawrence Berkeley National Laboratory, Stockholm University, the University of Tokyo and the University of Delaware.