Molecular Graphene Heralds New Era of 'Designer Electrons'
Menlo Park, Calif.
Menlo Park, Calif. — Researchers from Stanford University and the U.S. Department of Energy’s SLAC National Accelerator Laboratory have created the first-ever system of “designer electrons” – exotic variants of ordinary electrons with tunable properties that may ultimately lead to new types of materials and devices.
“The behavior of electrons in materials is at the heart of essentially all of today’s technologies,” said Hari Manoharan, associate professor of physics at Stanford and a member of SLAC’s Stanford Institute for Materials and Energy Sciences, who led the research. “We’re now able to tune the fundamental properties of electrons so they behave in ways rarely seen in ordinary materials.”
Their first examples, reported today in Nature, were hand-crafted, honeycomb-shaped structures inspired by graphene, a pure form of carbon that has been widely heralded for its potential in future electronics. Initially, the electrons in this structure had graphene-like properties; for example, unlike ordinary electrons, they had no mass and traveled as if they were moving at the speed of light in a vacuum. But researchers were then able to tune these electrons in ways that are difficult to do in real graphene.
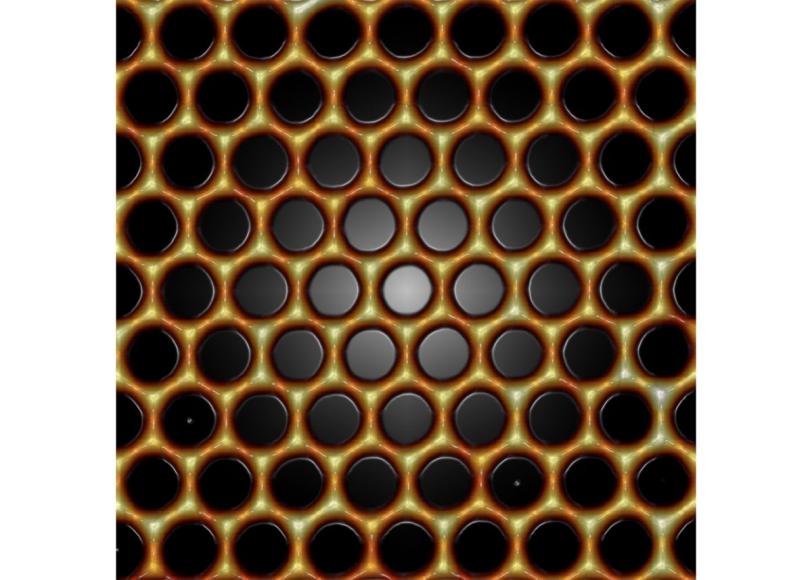
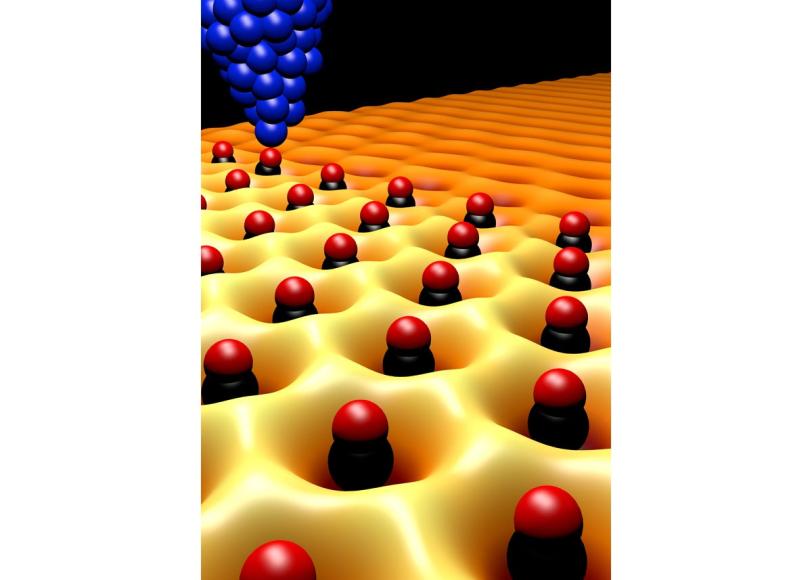
To make the structure, which Manoharan calls molecular graphene, the scientists use a scanning tunneling microscope to place individual carbon monoxide molecules on a perfectly smooth copper surface. The carbon monoxide repels the free-flowing electrons on the copper surface and forces them into a honeycomb pattern, where they behave like graphene electrons.
To tune the electrons’ properties, the researchers repositioned the carbon monoxide molecules on the surface; this changed the symmetry of the electron flow. In some configurations, electrons acted as if they had been exposed to a magnetic or electric field. In others, researchers were able to finely tune the density of electrons on the surface by introducing defects or impurities. By writing complex patterns that mimicked changes in carbon-carbon bond lengths and strengths in graphene, the researchers were able to restore the electrons’ mass in small, selected areas.
“One of the wildest things we did was to make the electrons think they are in a huge magnetic field when, in fact, no real field had been applied,”Manoharan said. Guided by the theory developed by co-author Francisco Guinea of Spain, the Stanford team calculated the positions where carbon atoms in graphene should be to make its electrons believe they were being exposed to magnetic fields ranging from zero to 60 Tesla, more than 30 percent higher than the strongest continuous magnetic field ever achieved on Earth. The researchers then moved carbon monoxide molecules to steer the electrons into precisely those positions, and the electrons responded by behaving exactly as predicted – as if they had been exposed to a real field.
“Our new approach is a powerful new test bed for physics,” Manoharan said. “Molecular graphene is just the first in a series of possible designer structures. We expect that our research will ultimately identify new nanoscale materials with useful electronic properties.”
Additional authors included Kenjiro K. Gomes, Warren Mar and Wonhee Ko of the Stanford Institute for Material and Energy Sciences. Francisco Guinea is a researcher at the Madrid Materials Science Institute. The research was supported by the U.S. Department of Energy’s Office of Basic Energy Sciences, the National Science Foundation and the Spanish Ministry of Science & Innovation.

Researchers Create 'Designer Electrons'
Stanford News Service
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, California, SLAC is operated by Stanford University for the U.S. Department of Energy Office of Science. To learn more, please visit www.slac.stanford.edu.
DOE’s Office of Science is the largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.