SSRL Researchers Show How Iron Activates Oxygen in Living Things
Oxygen performs many key functions in the body’s internal chemistry, but the life-sustaining molecule can’t do its job alone.
By Helen Shen
Oxygen performs many key functions in the body’s internal chemistry, but the life-sustaining molecule can’t do its job alone. Now, SLAC researchers and their collaborators are learning more about how iron-containing enzymes help oxygen play nice with other molecules.
The team used high-powered X-rays from the Stanford Synchrotron Radiation Lightsource to capture the fine details of how these enzymes work. The results could have applications in medicine, energy production and industrial processes.
Led by Edward Solomon, a professor of photon science at SLAC and the Department of Chemistry at Stanford, Wonwoo Nam, of Ewha Womans University in South Korea, and Joan Valentine of the University of California, Los Angeles, the team reported its findings in the Oct. 27 issue of Nature.
“It’s what we’re all after: How does nature make these metal sites do this chemistry better than scientists can do in the labs?” Solomon said.
In its most abundant form, oxygen exists as a two-atom molecule, O2. Electronically speaking, O2 is “forbidden” from reacting with other biological molecules until it is split into two separate oxygen atoms.
Specialized enzymes, containing metallic elements like iron, drive this important preparatory step. The precise mechanism of oxygen activation by iron complexes has long eluded researchers, in part because the reaction—which proceeds through multiple intermediate stages—happens in mere fractions of a second.
Researchers recently captured all three of the intermediate structures that one iron complex morphs into as it cleaves the O2 bond – including one wily intermediate that exists for less than 2 milliseconds before converting into a different form.
They used a specialized instrument at the SSRL to determine the electronic and geometric structure of each intermediate stage. Chemical tests at Ewha Womans University further revealed that these iron-based intermediates are versatile chemical catalysts, able to react with both electron-rich and electron-deficient molecules.
This is the first time researchers have so fully characterized this type of iron- and oxygen-containing molecule — called non-heme iron because it lacks the heme group for which the iron-containing molecule hemoglobin that carries oxygen in red blood cells is named.
Solomon says the newly discovered reaction mechanism could help scientists better understand diseases of non-heme iron enzymes, such as phenylketonuria. The disease, which prevents breakdown of the amino acid phenylalanine, can cause developmental defects in babies and lasting health problems for adults.
Other more distant applications may affect energy production and industrial processes that use similar chemistry. One day, Solomon says, industrial chemists may find a way to co-opt the non-heme iron enzyme reaction to drive reactions more quickly and cheaply.
Other co-authors on the paper, "Structure and reactivity of a mononuclear non-haem iron(III)–peroxo complex," include Stanford Photon Science Professors Britt Hedman, who is also science director of SSRL, and Keith Hodgson, SLAC's chief research officer and a professor in Stanford's chemistry department as well.
Helen Shen is a science communications intern at SLAC National Accelerator Laboratory.
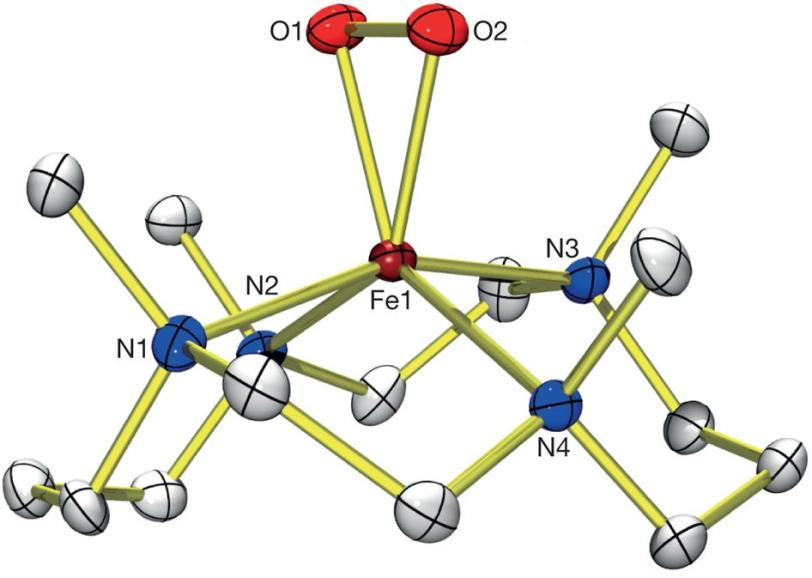
(Image courtesy of Jaeheung Cho, et. al.)